Lecture "Chemical Bonding"
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Physical and Analytical Electrochemistry: the Fundamental
Electrochemical Systems The simplest and traditional electrochemical process occurring at the boundary between an electronically conducting phase (the electrode) and an ionically conducting phase (the electrolyte solution), is the heterogeneous electro-transfer step between the electrode and the electroactive species of interest present in the solution. An example is the plating of nickel. Ni2+ + 2e- → Ni Physical and Analytical The interface is where the action occurs but connected to that central event are various processes that can Electrochemistry: occur in parallel or series. Figure 2 demonstrates a more complex interface; it represents a molecular scale snapshot The Fundamental Core of an interface that exists in a fuel cell with a solid polymer (capable of conducting H+) as an electrolyte. of Electrochemistry However, there is more to an electrochemical system than a single by Tom Zawodzinski, Shelley Minteer, interface. An entire circuit must be made and Gessie Brisard for measurable current to flow. This circuit consists of the electrochemical cell plus external wiring and circuitry The common event for all electrochemical processes is that (power sources, measuring devices, of electron transfer between chemical species; or between an etc). The cell consists of (at least) electrode and a chemical species situated in the vicinity of the two electrodes separated by (at least) one electrolyte solution. Figure 3 is electrode, usually a pure metal or an alloy. The location where a schematic of a simple circuit. Each the electron transfer reactions take place is of fundamental electrode has an interface with a importance in electrochemistry because it regulates the solution. Electrons flow in the external behavior of most electrochemical systems. -
Bond Energies in Oxide Systems: Calculated and Experimental Data
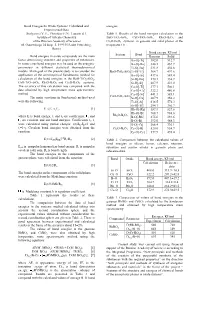
Bond Energies In Oxide Systems: Calculated and energies. Experimental Data Stolyarova V. L., Plotnikov E.N., Lopatin S.I. Table 1. Results of the bond energies calculation in the Institute of Silicate Chemistry BaO-TiO 2-SiO 2, CaO-TiO 2-SiO 2, Rb 2O-B2O3 and of the Russian Academy of Sciences Cs 2O-B2O3 systems. in gaseous and solid phases at the ul. Odoevskogo 24 korp. 2, 199155 Sankt Petersburg, temperature 0 ¥ . Russia Bond energy, KJ/mol System Bond Bond energies in oxide compounds are the main Gaseous Solid factor determining structure and properties of substances. Ba-O[-Ti] 382.0 512.7 In some cases bond energies may be used as the energetic Ba-O[-Ba] 348.5 467.7 parameters in different statistical thermodynamical Ti-O[-Ba] 331.8 450.6 models. Main goal of the present study is to consider the BaO-TiO 2-SiO 2 Ti-O[-Ti] 364.7 497.6 application of the semiempirical Sanderson's method for Ba-O[-Si] 437.6 585.4 calculation of the bond energies in the BaO-TiO 2-SiO 2, Si-O[-Ba] 270.3 334.7 CaO-TiO 2-SiO 2, Rb 2O-B2O3 and Cs 2O-B2O3 systems. Si-O[-Si] 407.9 421.0 The accuracy of this calculation was compared with the Ca-O[-Ti] 377.1 500.1 data obtained by high temperature mass spectrometric Ca-O[-Ca] 322.2 486.8 method. Ca-O[-Si] 441.1 585.1 CaO-TiO -SiO The main equations in Sanderson's method used 2 2 Si-O[-Ca] 287.9 358.2 were the following. -

Molecular Orbital Theory to Predict Bond Order • to Apply Molecular Orbital Theory to the Diatomic Homonuclear Molecule from the Elements in the Second Period
Skills to Develop • To use molecular orbital theory to predict bond order • To apply Molecular Orbital Theory to the diatomic homonuclear molecule from the elements in the second period. None of the approaches we have described so far can adequately explain why some compounds are colored and others are not, why some substances with unpaired electrons are stable, and why others are effective semiconductors. These approaches also cannot describe the nature of resonance. Such limitations led to the development of a new approach to bonding in which electrons are not viewed as being localized between the nuclei of bonded atoms but are instead delocalized throughout the entire molecule. Just as with the valence bond theory, the approach we are about to discuss is based on a quantum mechanical model. Previously, we described the electrons in isolated atoms as having certain spatial distributions, called orbitals, each with a particular orbital energy. Just as the positions and energies of electrons in atoms can be described in terms of atomic orbitals (AOs), the positions and energies of electrons in molecules can be described in terms of molecular orbitals (MOs) A particular spatial distribution of electrons in a molecule that is associated with a particular orbital energy.—a spatial distribution of electrons in a molecule that is associated with a particular orbital energy. As the name suggests, molecular orbitals are not localized on a single atom but extend over the entire molecule. Consequently, the molecular orbital approach, called molecular orbital theory is a delocalized approach to bonding. Although the molecular orbital theory is computationally demanding, the principles on which it is based are similar to those we used to determine electron configurations for atoms. -

Calculating Lattice Energies Using the Born-Haber Cycle an Extension Activity for AP Chemistry Students
Calculating Lattice Energies Using the Born-Haber Cycle An Extension Activity for AP Chemistry Students A particular set of equations known as a Born-Haber cycle demonstrates how chemists are able to use the first law of thermodynamics—that the energy of the universe is conserved in any chemical or physical change—to find an unknown energy value that is difficult or impossible to measure experimentally. Some energy quantities, such as the lattice energy of a mineral or the electron affinity of an atom, can be difficult to measure in the lab. Examining a Born-Haber cycle we see that there is more than one path to the formation of a substance in a particular state, and that if we use consistent definitions, an energy value that we seek can be calculated from energy values that we already know. The following exercise will help us see the way these energy values relate to one another, give us practice with their definitions and symbols, and deepen our insight to their meaning when we see them in other types of problems. Each physical or chemical change represented has: • an equation that represents a clearly defined physical or chemical change; • a definition of the particular type of energy change; • a symbol or abbreviation for the energy change equal to a value for the change in enthalpy (ΔH), the energy that is released or absorbed during the change, expressed in kJ/mol; and • a name by which that change in enthalpy is commonly known. To set up the equations in a Born-Haber cycle, cut out the cards for names, equations, defini- tions, and symbols with energy values. -

Astrochemistry in Galaxies of the Local Universe Authors: David S
Astrochemistry in Galaxies of the Local Universe Authors: David S. Meier (New Mexico Tech), Jean Turner (UCLA), An- thony Remijan (NRAO), Juergen Ott (NRAO) and Alwyn Wootten (NRAO) Contact Author: David S. Meier New Mexico Institute of Mining and Technology Phone: 575-835-5340 Fax: 575-835-5707 E-mail: [email protected] Science Frontier Area: Galactic Neighborhood Abstract To understand star formation in galaxies we must know the molecular fuel out of which the stars form. Telescopes coming online in the upcoming decade will enable us to understand the molecular ISM from a new and powerful global perspective. In this white paper we focus on the use of astrochemistry—the observation, analysis and theoretical modeling of many molecular lines and their correlations—to answer some of the outstanding questions regarding the evolution of star formation and galactic structure in nearby galaxies. The 2 mm spectral line survey of the nearby starburst spiral, NGC 253 (Martin et al. 2006), overlaid on the 2 MASS near infrared JHK color image. The spectrum shows the richness of the millimeter spectrum. 1 Introduction Galactic structure and its evolution across cosmic time is driven by the gaseous component of galaxies. Our knowledge of the gaseous universe beyond the Milky Way is sketchy at present. Least studied of all is molecular gas, the component most closely linked to star formation. How has the gaseous structure of galaxies changed through cosmic time? What is the nature of intergalactic gas and its interaction with galaxies? How do galaxies accrete gas to form disks, giant molecular clouds (GMCs), stars and star clusters? How are GMCs affected by their environment? What is the relative importance of secular evolution and impulsive dynamical interactions in the histories of different kinds of galaxies? In this white paper we focus on how to concretely address these questions through astrochemistry. -

The Theoretical Study on Interaction of Hydrogen with Single-Walled Boron Nitride Nanotubes
THE JOURNAL OF CHEMICAL PHYSICS 123, 114703 ͑2005͒ The theoretical study on interaction of hydrogen with single-walled boron nitride nanotubes. I. The reactive force field ReaxFFHBN development ͒ Sang Soo Han, Jeung Ku Kang, and Hyuck Mo Leea Department of Materials Science and Engineering, Korea Advanced Institute of Science and Technology (KAIST), Daejeon 305-701, Republic of Korea Adri C. T. van Duin and William A. Goddard III Materials and Process Simulation Center, California Institute of Technology, California 91125 ͑Received 2 November 2004; accepted 21 June 2005; published online 19 September 2005͒ We present a new reactive force field ReaxFFHBN derived to accurately model large molecular and condensed phase systems of H, B, and N atoms. ReaxFFHBN has been tested against quantum calculation data for B–H, B–B, and B–N bond dissociations and for H–B–H, B–N–B, and N–B–N bond angle strain energies of various molecular clusters. The accuracy of the developed ReaxFFHBN for B–N–H systems is also tested for ͑i͒ H–B and H–B bond energies as a function of out of plane ͑ ͒ ͑ ͒ ͑ ͒ in H–B NH2 3 and H–N BH2 3, respectively, ii the reaction energy for the B3N3H6 +H2 → ͑ ͒ B3N3H8, and iii crystal properties such as lattice parameters and equations of states for the hexagonal type ͑h-BN͒ with a graphite structure and for the cubic type ͑c-BN͒ with a zinc-blende structure. For all these systems, ReaxFFHBN gives reliable results consistent with those from quantum calculations as it describes well bond breaking and formation in chemical processes and physical properties. -

Session #9: Homework Solutions
Session #9: Homework Solutions Problem #1 Estimate the ionic radius of Cs+. The lattice energy of CsCl is 633 kJ/mol. For CsCl the Madelung constant, M, is 1.763, and the Born exponent, n, is 10.7. The ionic radius of Cl– is known to be 1.81 Å. Solution Mq q ⎛⎞1 E1 = 1 2 −= and r r+r lattice ⎜⎟ o Cs+ Cl− 4rπεoo⎝⎠ n Solve first for ro MqqN ⎛⎞1 1.763(1.6 x 10-19 ) 2 6.02 x 1023 ⎛ 1 ⎞ r1 = 12 Aν −− = 1 o ⎜⎟ -12 5 ⎜ ⎟ 4Eπεolattice⎝⎠ n 4π 8.85 x 10 6.33x10 ⎝10.7⎠ = 3.50 x 10-10 m = 3.50 Å = r+ r Cs+- Cl ∴ r = 3.50-1.81 = 1.69 Å Cs+ Problem #2 (a) CFCs have been implicated in ozone depletion. Show that when Freon 12 (CCl2F2) is exposed to ultraviolet radiation, the compound decomposes to produce chlorine. (b) Draw the Lewis structure of Freon 12 and indicate the polarities of each bond within this compound. (c) Determine the percent ionic character of the C--Cl and C--F bonds. DATA: Average Bond Energies (kJ/mol) Single Bonds Multiple Bonds H-H 435 C = C 610 F-F 155 C ≡ C 836 Cl-Cl 242 C-C 347 Solution (a) Chlorine will be liberated if the C-Cl bond breaks, therefore, compute its strength and show that ultraviolet photons have enough energy to break the bond. 2 ECCl−−− =+ E CCx9 E ClCl 6.3(χχC− Cl) = 347 x 242 + 96.3(2.55 - 3.16)2 = 326 x 103/6.02 x 1023 = 5.41 x 10-19 J/bond photon will break ∴this bond if Eph > Ebond ⇒ critical λ is hc 6.6 x 10-34 x 3 x 108 λ = = = 3.66 x 10-7 m -19 Ebond 5.41 x 10 which lies in the u.v. -

Guidelines for Terms Related to Chemical Speciation and Fractionation of Elements. Definitions, Structural Aspects, and Methodological Approaches
Pure Appl. Chem., Vol. 72, No. 8, pp. 1453–1470, 2000. © 2000 IUPAC INTERNATIONAL UNION FOR PURE AND APPLIED CHEMISTRY ANALYTICAL CHEMISTRY DIVISION COMMISSION ON MICROCHEMICAL TECHNIQUES AND TRACE ANALYSIS CHEMISTRY AND THE ENVIRONMENT DIVISION COMMISSION ON FUNDAMENTAL ENVIRONMENTAL CHEMISTRY CHEMISTRY AND HUMAN HEALTH DIVISION CLINICAL CHEMISTRY SECTION, COMMISSION ON TOXICOLOGY GUIDELINES FOR TERMS RELATED TO CHEMICAL SPECIATION AND FRACTIONATION OF ELEMENTS. DEFINITIONS, STRUCTURAL ASPECTS, AND METHODOLOGICAL APPROACHES (IUPAC Recommendations 2000) Prepared for publication by DOUGLAS M. TEMPLETON1, FREEK ARIESE2, RITA CORNELIS3*, LARS-GÖRAN DANIELSSON4, HERBERT MUNTAU5, HERMAN P. VAN LEEUWEN6, AND RYSZARD ŁOBIŃSKI7 1Department of Laboratory Medicine and Pathobiology, University of Toronto, Canada; 2Department of Analytical Chemistry and Applied Spectroscopy, Vrije Universiteit Amsterdam, the Netherlands; 3Laboratory for Analytical Chemistry, Faculty of Sciences, University of Gent, Belgium; 4Department of Analytical Chemistry, Royal Institute of Technology, Stockholm, Sweden; 5Environmental Institute, Joint Research Centre Ispra, (VA) Italy; 6Department of Physical Chemistry and Colloid Science, Wageningen Agricultural University, the Netherlands; 7CNRS UMR 5034, Hélioparc, Pau, France, and Department of Chemistry, Warsaw University of Technology, Warszawa, Poland *Corresponding author: Laboratory for Analytical Chemistry, RUG, Proeftuinstraat 86, B-9000 Gent, Belgium; E-mail: [email protected] Republication or reproduction of this report or its storage and/or dissemination by electronic means is permitted without the need for formal IUPAC permission on condition that an acknowledgment, with full reference to the source along with use of the copyright symbol ©, the name IUPAC, and the year of publication, are prominently visible. Publication of a translation into another language is subject to the additional condition of prior approval from the relevant IUPAC National Adhering Organization. -

Stoichiometry of Chemical Reactions 175
Chapter 4 Stoichiometry of Chemical Reactions 175 Chapter 4 Stoichiometry of Chemical Reactions Figure 4.1 Many modern rocket fuels are solid mixtures of substances combined in carefully measured amounts and ignited to yield a thrust-generating chemical reaction. (credit: modification of work by NASA) Chapter Outline 4.1 Writing and Balancing Chemical Equations 4.2 Classifying Chemical Reactions 4.3 Reaction Stoichiometry 4.4 Reaction Yields 4.5 Quantitative Chemical Analysis Introduction Solid-fuel rockets are a central feature in the world’s space exploration programs, including the new Space Launch System being developed by the National Aeronautics and Space Administration (NASA) to replace the retired Space Shuttle fleet (Figure 4.1). The engines of these rockets rely on carefully prepared solid mixtures of chemicals combined in precisely measured amounts. Igniting the mixture initiates a vigorous chemical reaction that rapidly generates large amounts of gaseous products. These gases are ejected from the rocket engine through its nozzle, providing the thrust needed to propel heavy payloads into space. Both the nature of this chemical reaction and the relationships between the amounts of the substances being consumed and produced by the reaction are critically important considerations that determine the success of the technology. This chapter will describe how to symbolize chemical reactions using chemical equations, how to classify some common chemical reactions by identifying patterns of reactivity, and how to determine the quantitative relations between the amounts of substances involved in chemical reactions—that is, the reaction stoichiometry. 176 Chapter 4 Stoichiometry of Chemical Reactions 4.1 Writing and Balancing Chemical Equations By the end of this section, you will be able to: • Derive chemical equations from narrative descriptions of chemical reactions. -

Real Space in Situ Bond Energies: Toward a Consistent
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Repositorio Institucional de la Universidad de Oviedo Real space in situ bond energies: toward a consistent energetic definition of bond strength D. Menéndez-Crespo, A. Costales, E. Francisco, and A. Martín Pendása∗ June 20, 2018 Abstract A rigorous definition of intrinsic bond strength based on the partitioning of a molecule into real space fragments is presented. Using the domains provided by the quantum theory of atoms in molecules (QTAIM) together with the interacting quantum atoms (IQA) energetic decomposition, we show how an in situ bond strength, matching all the requirements of an intrinsic bond energy, can be defined between each pair of fragments. Total atomization or fragmentation energies are shown to be equal to the sum of these in situ bond energies (ISBEs) if the energies of the fragments are measured with respect to their in-the-molecule state. These energies usually lie above the ground state of the isolated fragments by quantities identified with the standard frag- ment relaxation or deformation energies, which are also provided by the protocol. Deformation energies bridge dissociation energies with ISBEs, and can be dissected using well-known tools of real space theories of chemical bonding. Similarly, ISBEs can be partitioned into ionic and covalent contributions, and this feature adds to the chemical appeal of the procedure. All the energetic quantities examined are observable and amenable, in principle, to experimental deter- mination. Several systems, exemplifying the role of each energetic term herein presented are used to show the power of the approach. -

Chemical Bonding and Molecular Structure
100 CHEMISTRY UNIT 4 CHEMICAL BONDING AND MOLECULAR STRUCTURE Scientists are constantly discovering new compounds, orderly arranging the facts about them, trying to explain with the existing knowledge, organising to modify the earlier views or After studying this Unit, you will be evolve theories for explaining the newly observed facts. able to • understand KÖssel-Lewis approach to chemical bonding; • explain the octet rule and its Matter is made up of one or different type of elements. limitations, draw Lewis Under normal conditions no other element exists as an structures of simple molecules; independent atom in nature, except noble gases. However, • explain the formation of different a group of atoms is found to exist together as one species types of bonds; having characteristic properties. Such a group of atoms is • describe the VSEPR theory and called a molecule. Obviously there must be some force predict the geometry of simple which holds these constituent atoms together in the molecules; molecules. The attractive force which holds various • explain the valence bond constituents (atoms, ions, etc.) together in different approach for the formation of chemical species is called a chemical bond. Since the covalent bonds; formation of chemical compounds takes place as a result • predict the directional properties of combination of atoms of various elements in different of covalent bonds; ways, it raises many questions. Why do atoms combine? Why are only certain combinations possible? Why do some • explain the different types of hybridisation involving s, p and atoms combine while certain others do not? Why do d orbitals and draw shapes of molecules possess definite shapes? To answer such simple covalent molecules; questions different theories and concepts have been put forward from time to time. -

1 Introductory Lecture/S the Distribution, Fate and Transformations of Chemical Species in Water → Physical and Chemical Prope
CHEM 301: Aqueous Environmental Chemistry Introductory Lecture/s The Distribution, Fate and Transformations of chemical species in Water Physical and Chemical properties of dissolved and suspended species Examples of aqueous environmental chemistry: Thames River, UK: 1860’s Great Lakes, Canada: 1970’s Polar concentrations: 1990 - 2000’s Emerging issues: What this course is NOT (however, these are related and will come up) Limnology/Microbial Ecology Chemical Analysis Economics/Political science Environmental Studies What this course IS about: The chemistry of water and the species found in water (natural and anthropogenic) • What are they? • How long do they stay? • Where do they go? • How are they transformed? Chemical changes and interactions are governed by principles of PHYSICAL CHEMISTRY: Thermodynamics and Kinetics Thermodynamics describe EQUILIBRIA phenomena predict chemical concs and solubility, acid base and redox behaviour Kinetics describe RATES predict how fast reactions occur and stationary state concs introductory lecture 2012.doc 1 CHEM 301: Aqueous Environmental Chemistry WATER QUANTITY AND QUALITY Quantity: Water use and demand follow population and agriculture. Most of which is concentrated in arid and semi-arid regions globally. In temperate climates, demand increases as supply decreases. • Ogallala aquifer (one of the largest in US) is being depleted ~1000X faster than re-charge • Sahara aquifer is being drained with ~zero recharge (e.g., water mining) • Mexico city is collapsing as aquifer is drained • S. California