321444 1 En Bookbackmatter 533..564
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Looking Forward: Long-Term Perspectives on Recovery, Risk-Reduction, and Research
Looking forward: Long-term Perspectives on Recovery, Risk-Reduction, and Research Kim A. Gorgens, Ph.D., ABPP Graduate School of Professional Psychology/GSPP [email protected] @bubblewrapbrain •Chronic Symptom Management (Recovery) •Aging with a Vulnerable Brain (Risk-Reduction) •Emerging Technologies (Research) Mounting Evidence for a Lifetime of Change • “The propensity for experience dependent plasticity throughout life can be more or less potentiated by diverse factors including individual genetic, cellular, molecular, and environmental differences. These findings have lead us to understand that the rules that regulate plasticity are not only more intrinsically variable than were previously thought, but can also be shaped in mature brains.” • “As with many medical and health related fields where personalized and precision medicine are increasingly becoming mainstream, neurotherapeutic interventions targeting mechanisms of plasticity and cognition should also follow an individualized approach by harnessing individual differences to best utilize the brain’s innate capacity to change.” • See Patrice Voss, Maryse E. Thomas, J. Miguel Cisneros-Franco, & Étienne de Villers-Sidani. (2017). Dynamic Brains and the Changing Rules of Neuroplasticity: Implications for Learning and Recovery. Frontiers in Psychology, 8. A Trio of Challenges Post-Traumatic Headaches (PTH or PTHA) • Chronic post-traumatic headache=12+ months after injury • Rates reach up to 95% • 71% after moderate/severe TBI and 91% after mild TBI (mTBI) at 1 year (Lucas, -

Creation: Believe It Or Not*
TMSJ 13/1 (Spring 2002) 5-32 CREATION: BELIEVE IT OR NOT* John MacArthur President and Professor of Pastoral Ministries Naturalism has replaced Christianity as the main religion of the Western world. Though the teaching that natural evolutionary processes can account of the origin of all living species has never been proven, that teaching is central to the philosophy that now dominates Western scholarly thinking. Even evangelicals have become less willing to defend the early chapters of Genesis against the encroach- ments of evolutionary thought, although in actuality affirming an “old earth” theory and remaining evangelical is an inconsistency. A “framework” approach to those chapters does not square with a consistent hermeneutical approach to Scripture, because the first chapter of Genesis teaches that God created the world in a normal week of seven days. The purpose of evolution is to explain away the God of the Bible. The absurd teaching of the Big Bang theory of evolution is that nobody times nothing equals everything. It is a theory that raises an almost endless array of unsolvable problems. It is degrading to humanity, hostile to reasons, and antithetical to the truth that God has revealed. When one starts adapting the Word of God to fit scientific theories based on naturalistic beliefs, he has begun his journey on the road to skepticism. * * * * * Introduction Thanks to the theory of evolution, naturalism is now the dominant religion of modern society. Less than a century and a half ago, Charles Darwin popularized the credo for this secular religion with his book The Origin of Species. -

Identificação De Textos Em Imagens CAPTCHA Utilizando Conceitos De
Identificação de Textos em Imagens CAPTCHA utilizando conceitos de Aprendizado de Máquina e Redes Neurais Convolucionais Relatório submetido à Universidade Federal de Santa Catarina como requisito para a aprovação da disciplina: DAS 5511: Projeto de Fim de Curso Murilo Rodegheri Mendes dos Santos Florianópolis, Julho de 2018 Identificação de Textos em Imagens CAPTCHA utilizando conceitos de Aprendizado de Máquina e Redes Neurais Convolucionais Murilo Rodegheri Mendes dos Santos Esta monografia foi julgada no contexto da disciplina DAS 5511: Projeto de Fim de Curso e aprovada na sua forma final pelo Curso de Engenharia de Controle e Automação Prof. Marcelo Ricardo Stemmer Banca Examinadora: André Carvalho Bittencourt Orientador na Empresa Prof. Marcelo Ricardo Stemmer Orientador no Curso Prof. Ricardo José Rabelo Responsável pela disciplina Flávio Gabriel Oliveira Barbosa, Avaliador Guilherme Espindola Winck, Debatedor Ricardo Carvalho Frantz do Amaral, Debatedor Agradecimentos Agradeço à minha mãe Terezinha Rodegheri, ao meu pai Orlisses Mendes dos Santos e ao meu irmão Camilo Rodegheri Mendes dos Santos que sempre estiveram ao meu lado, tanto nos momentos de alegria quanto nos momentos de dificuldades, sempre me deram apoio, conselhos, suporte e nunca duvidaram da minha capacidade de alcançar meus objetivos. Agradeço aos meus colegas Guilherme Cornelli, Leonardo Quaini, Matheus Ambrosi, Matheus Zardo, Roger Perin e Victor Petrassi por me acompanharem em toda a graduação, seja nas disciplinas, nos projetos, nas noites de estudo, nas atividades extracurriculares, nas festas, entre outros desafios enfrentados para chegar até aqui. Agradeço aos meus amigos de infância Cássio Schmidt, Daniel Lock, Gabriel Streit, Gabriel Cervo, Guilherme Trevisan, Lucas Nyland por proporcionarem momentos de alegria mesmo a distância na maior parte da caminhada da graduação. -

Watson Daniel.Pdf (5.294Mb)
Source Code Stylometry and Authorship Attribution for Open Source by Daniel Watson A thesis presented to the University of Waterloo in fulfillment of the thesis requirement for the degree of Master of Mathematics in Computer Science Waterloo, Ontario, Canada, 2019 c Daniel Watson 2019 Author's Declaration I hereby declare that I am the sole author of this thesis. This is a true copy of the thesis, including any required final revisions, as accepted by my examiners. I understand that my thesis may be made electronically available to the public. ii Abstract Public software repositories such as GitHub make transparent the development history of an open source software system. Source code commits, discussions about new features and bugs, and code reviews are stored and carefully attributed to the appropriate developers. However, sometimes governments may seek to analyze these repositories, to identify citi- zens who contribute to projects they disapprove of, such as those involving cryptography or social media. While developers who seek anonymity may contribute under assumed identi- ties, their body of public work may be characteristic enough to betray who they really are. The ability to contribute anonymously to public bodies of knowledge is extremely impor- tant to the future of technological and intellectual freedoms. Just as in security hacking, the only way to protect vulnerable individuals is by demonstrating the means and strength of available attacks so that those concerned may know of the need and develop the means to protect themselves. In this work, we present a method to de-anonymize source code contributors based on the authors' intrinsic programming style. -

Use Style: Paper Title
Volume 3, Issue 10, October – 2018 International Journal of Innovative Science and ResearchTechnology ISSN No:-2456-2165 How Blockchain can be used for Digitization of Human Consciousness Alastair Smith MS in Artificial Intelligence, Northwestern University New York, United States Abstract:- This article presents empirical evidence "sleep" of death, or bringing the bodies back to life and then collected from experts and professionals in the field of transferring them the memory downloaded. Recently the news digitization of minds and virtual reality about the potential has been spread that the American start-up Nectome has role that blockchain technology could play in the started a project of cryopreservation to the mind, waiting for digitization of human consciousness. The results of the the scientists to develop a system to digitize the thoughts and survey and secondary research indicate that blockchain recreate them on the computer. But in order for the technology can be used to enhance the security and privacy metamorphosis to succeed, the process must begin when the of digitized minds. The study shows evidence with brain is still alive, or at most a few minutes after death. supported arguments to the role that blockchain Around 25 people have already booked for the procedure [1]. technology could play in terms of prevention of attacks, data integrity, availability, confidentiality, operational B. Mind uploading, the biological brain mapped and copied security and privacy. The transfer of the mind or brain emulation is the project that involves the hypothetical process of transferring or Keywords:- Digitization of human consciousness,Blockchain copying a conscious mind from a brain to a non-biological technology; Security; Privacy; substrate. -

Vikas Sindhwani Google May 17-19, 2016 2016 Summer School on Signal Processing and Machine Learning for Big Data
Real-time Learning and Inference on Emerging Mobile Systems Vikas Sindhwani Google May 17-19, 2016 2016 Summer School on Signal Processing and Machine Learning for Big Data Abstract: We are motivated by the challenge of enabling real-time "always-on" machine learning applications on emerging mobile platforms such as next-generation smartphones, wearable computers and consumer robotics systems. On-device models in such settings need to be highly compact, and need to support fast, low-power inference on specialized hardware. I will consider the problem of building small-footprint non- linear models based on kernel methods and deep learning techniques, for on-device deployments. Towards this end, I will give an overview of various techniques, and introduce new notions of parsimony rooted in the theory of structured matrices. Such structured matrices can be used to recycle Gaussian random vectors in order to build randomized feature maps in sub-linear time for approximating various kernel functions. In the deep learning context, low-displacement structured parameter matrices admit fast function and gradient evaluation. I will discuss how such compact nonlinear transforms span a rich range of parameter sharing configurations whose statistical modeling capacity can be explicitly tuned along a continuum from structured to unstructured. I will present empirical results on mobile speech recognition problems, and image classification tasks. I will also briefly present some basics of TensorFlow: a open-source library for numerical computations on data flow graphs. Tensorflow enables large-scale distributed training of complex machine learning models, and their rapid deployment on mobile devices. Bio: Vikas Sindhwani is Research Scientist in the Google Brain team in New York City. -

Warfare in a Fragile World: Military Impact on the Human Environment
Recent Slprt•• books World Armaments and Disarmament: SIPRI Yearbook 1979 World Armaments and Disarmament: SIPRI Yearbooks 1968-1979, Cumulative Index Nuclear Energy and Nuclear Weapon Proliferation Other related •• 8lprt books Ecological Consequences of the Second Ihdochina War Weapons of Mass Destruction and the Environment Publish~d on behalf of SIPRI by Taylor & Francis Ltd 10-14 Macklin Street London WC2B 5NF Distributed in the USA by Crane, Russak & Company Inc 3 East 44th Street New York NY 10017 USA and in Scandinavia by Almqvist & WikseH International PO Box 62 S-101 20 Stockholm Sweden For a complete list of SIPRI publications write to SIPRI Sveavagen 166 , S-113 46 Stockholm Sweden Stoekholol International Peace Research Institute Warfare in a Fragile World Military Impact onthe Human Environment Stockholm International Peace Research Institute SIPRI is an independent institute for research into problems of peace and conflict, especially those of disarmament and arms regulation. It was established in 1966 to commemorate Sweden's 150 years of unbroken peace. The Institute is financed by the Swedish Parliament. The staff, the Governing Board and the Scientific Council are international. As a consultative body, the Scientific Council is not responsible for the views expressed in the publications of the Institute. Governing Board Dr Rolf Bjornerstedt, Chairman (Sweden) Professor Robert Neild, Vice-Chairman (United Kingdom) Mr Tim Greve (Norway) Academician Ivan M£ilek (Czechoslovakia) Professor Leo Mates (Yugoslavia) Professor -
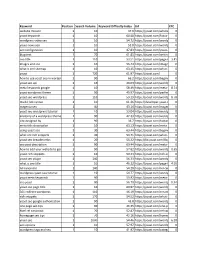
Keyword Position Search Volume Keyword Difficulty Index Url CPC
Keyword Position Search Volume Keyword Difficulty Index Url CPC website mission 1 10 47.9 https://yoast.com/whats-your-mission/0 yoast keywords 1 10 60.48 https://yoast.com/focus-keyword/0 wordpress video seo 1 10 54.72 https://yoast.com/wordpress/plugins/video-seo/0 yoast news seo 1 10 51.8 https://yoast.com/wordpress/plugins/news-seo/0 seo configuration 1 10 47.83 https://yoast.com/yoast-seo-configuration-wizard/0 blog text 1 70 61.85 https://yoast.com/writing-blog-text-objective-blogpost/0 seo title 1 110 53.51 https://yoast.com/page-titles-seo/3.45 images and seo 1 10 56.43 https://yoast.com/image-seo/ 0 what is xml sitemap 1 10 63.45 https://yoast.com/what-is-an-xml-sitemap-and-why-should-you-have-one/0 yoast 1 720 61.97 https://yoast.com/ 0 how to use yoast seo in wordpress 1 90 66.2 https://yoast.com/beginners-guide-yoast-seo/0 yoast seo api 1 10 49.03 https://yoast.com/wordpress/plugins/seo/api/0 meta keywords google 1 10 58.49 https://yoast.com/meta-keywords/8.74 yoast wordpress theme 1 50 49.97 https://yoast.com/perfect-wordpress-theme/0 yoast seo wordpress 1 110 59.29 https://yoast.com/wordpress/plugins/seo/8.78 mailto link syntax 1 10 61.36 https://developer.yoast.com/guide-mailto-links/0 magento seo 1 40 43.14 https://yoast.com/magento-seo/0 yoast seo wordpress tutorial 1 10 53.04 https://yoast.com/wordpress-seo/0 anatomy of a wordpress theme 1 90 47.33 https://yoast.com/wordpress-theme-anatomy/0 site designed by 1 90 36.7 https://yoast.com/footer-design-by-links/0 meta title description 1 10 63.13 https://yoast.com/meta-descriptions/0 -
Google for Education National Parks VR Google Expeditions Google Arts & Culture Be Internet Awesome Made with Code CS First Google Science Fair
Google For Education National Parks VR Google Expeditions Google Arts & Culture Be Internet Awesome Made with Code CS First Google Science Fair Learn how tools built for teaching and learning, Discover the hidden worlds of our Take a field trip to virtually anywhere (even Explore works of art and stories from around Teach learners the fundamentals of digital Make the connection between coding and Learn the basics of Computer Science using the Create projects that show how Science, like Classroom, G Suite for Education, and National Parks on ranger-guided trips places school buses can’t go) using immersive the world with 360° tours of exhibits in over 70 safety and citizenship so they can be safe, creativity, empowering girls to engage with block-based programming language, Scratch. Technology, Engineering, and Math can be used Chromebooks, can increase engagement through virtual reality. AR and VR technology. different countries. confident explorers of the online world. technology and bring their ideas to life. to impact the world around us. and inspire curiosity. Google for National Google Google Arts Education Parks VR Expeditions & Culture Discover the hidden worlds of Explore works of art and stories Tools built for teaching and learning. Field trips to virtually anywhere. our National Parks. from around the world. Over 80 million teachers and students around the world Take a ranger-guided journey through the glaciers of What if you could journey to the surface of Mars or From The Metropolitan Museum of Art to Nairobi’s communicate and collaborate using Classroom and Alaska’s Kenai Fjords or get up close with active climb to the top of Machu Picchu without ever leaving Kenya National Archives, Google Arts & Culture offers G Suite for Education, while Chromebooks are the most volcanoes in Hawai’i. -

Mashing-Up Maps Google Geo Services and the Geography Of
Mashing-up Maps Google Geo Services and the Geography of Ubiquity Craig M. Dalton A dissertation submitted to the faculty of the University of North Carolina at Chapel Hill in partial fulfillment of the requirements for the degree of Doctor in Philosophy in the Department of Geography. Chapel Hill 2012 Approved by: Dr. Scott Kirsch Dr. Banu Gokariksel Dr. Kenneth Hillis Dr. John Pickles Dr. Sarah Sharma © 2012 Craig M. Dalton ALL RIGHTS RESERVED ii Abstract CRAIG DALTON: Mashing-up Maps: Google Geo Services and the Geography of Ubiquity (Under the direction of Scott Kirsch) How are Google geo services such as Google Maps and Google Earth shaping ways of seeing the world? These geographic ways of seeing are part of an influential and problematic geographic discourse. This discourse reaches hundreds of millions of people, though not all have equal standing. It empowers many people to make maps on the geoweb, but within the limits of Google’s business strategy. These qualities, set against the state-centeredness of mapmaking over the last six hundred years, mark the Google geo discourse as something noteworthy, a consumer-centered mapping in a popular geographic discourse. This dissertation examines the Google geo discourse through its social and technological history, Google’s role in producing and limiting the discourse, and the subjects who make and use these maps. iii Acknowledgements This dissertation was only possible with the help of a large number of people. I owe each a debt of gratitude. Chief among them is a fantastic advisor, Scott Kirsch. His patience, grace, and good criticism saw me through the trials of graduate school. -

Desired Ground Zeroes: Nuclear Imagination and the Death Drive Calum Lister Matheson a Dissertation Submitted to the Faculty At
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Carolina Digital Repository Desired Ground Zeroes: Nuclear Imagination and the Death Drive Calum Lister Matheson A dissertation submitted to the faculty at the University of North Carolina at Chapel Hill in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Department of Communication Studies. Chapel Hill 2015 Approved by: Carole Blair Ken Hillis Chris Lundberg Todd Ochoa Sarah Sharma © 2015 Calum Lister Matheson ALL RIGHTS RESERVED ii ABSTRACT Calum Lister Matheson: Desired Ground Zeroes: Nuclear Imagination and the Death Drive (Under the direction of Chris Lundberg and Sarah Sharma) A wide variety of cultural artefacts related to nuclear warfare are examined to highlight continuity in the sublime’s mix of horror and fascination. Schemes to use nuclear explosions for peaceful purposes embody the godlike structural positions of the Bomb for Americans in the early Cold War. Efforts to mediate the Real of the Bomb include nuclear simulations used in wargames and their civilian offshoots in videogames and other media. Control over absence is examined through the spatial distribution of populations that would be sacrificed in a nuclear war and appeals to overarching rationality to justify urban inequality. Control over presence manifests in survivalism, from Cold War shelter construction to contemporary “doomsday prepping” and survivalist novels. The longstanding cultural ambivalence towards nuclear war, coupled with the manifest desire to experience the Real, has implications for nuclear activist strategies that rely on democratically-engaged publics to resist nuclear violence once the “truth” is made clear. -

Health Options Foreclosed: How the FDA Denies Americans the Benefits
Health Options Foreclosed How the FDA Denies Americans the Benefits of Medical Research Richard Williams, Marc Joffe, and Ariel Slonim September 2016 MERCATUS WORKING PAPER Richard Williams, Marc Joffe, and Ariel Slonim. “Health Options Foreclosed: How the FDA Denies Americans the Benefits of Medical Research.” Mercatus Working Paper, Mercatus Center at George Mason University, Arlington, VA, September 2016. Abstract In recent decades, the Food and Drug Administration (FDA) has assumed increasing premarket authority for drugs and devices. Given how the FDA’s regulatory stance has inhibited breakthroughs in the development of medical products, it appears that the agency will stand in the way of emerging technologies such as nanotechnology, synthetic biology, nanorobotics, virally delivered telomerase, and cellular therapy. These new technologies represent not only the means to prevent and cure diseases, but also the key to helping people live longer and healthier lives. We conclude, therefore, that an incremental approach to reform—one that would keep the FDA as the sole arbiter of new medical technologies—is unlikely to work. Rather, we think that a regulatory system based on competitive market approval of drugs and devices is more likely to strike the appropriate benefit-risk balance, including that inherent in the compassionate use of experimental medical treatments. JEL codes: H11, I18, L65 Keywords: FDA, regulation, drugs, biotechnology, pharmaceuticals Author Affiliation and Contact Information Richard Williams Director for the Regulatory Studies Program Mercatus Center at George Mason University [email protected] Marc Joffe Principal Consultant Public Sector Credit Solutions [email protected] Ariel Slonim MA Fellow, Mercatus Center All studies in the Mercatus Working Paper series have followed a rigorous process of academic evaluation, including (except where otherwise noted) at least one double-blind peer review.