Carbohydrate Chemistry
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pentose PO4 Pathway, Fructose, Galactose Metabolism.Pptx
Pentose PO4 pathway, Fructose, galactose metabolism The Entner Doudoroff pathway begins with hexokinase producing Glucose 6 PO4 , but produce only one ATP. This pathway prevalent in anaerobes such as Pseudomonas, they doe not have a Phosphofructokinase. The pentose phosphate pathway (also called the phosphogluconate pathway and the hexose monophosphate shunt) is a biochemical pathway parallel to glycolysis that generates NADPH and pentoses. While it does involve oxidation of glucose, its primary role is anabolic rather than catabolic. There are two distinct phases in the pathway. The first is the oxidative phase, in which NADPH is generated, and the second is the non-oxidative synthesis of 5-carbon sugars. For most organisms, the pentose phosphate pathway takes place in the cytosol. For each mole of glucose 6 PO4 metabolized to ribulose 5 PO4, 2 moles of NADPH are produced. 6-Phosphogluconate dh is not only an oxidation step but it’s also a decarboxylation reaction. The primary results of the pathway are: The generation of reducing equivalents, in the form of NADPH, used in reductive biosynthesis reactions within cells (e.g. fatty acid synthesis). Production of ribose-5-phosphate (R5P), used in the synthesis of nucleotides and nucleic acids. Production of erythrose-4-phosphate (E4P), used in the synthesis of aromatic amino acids. Transketolase and transaldolase reactions are similar in that they transfer between carbon chains, transketolases 2 carbon units or transaldolases 3 carbon units. Regulation; Glucose-6-phosphate dehydrogenase is the rate- controlling enzyme of this pathway. It is allosterically stimulated by NADP+. The ratio of NADPH:NADP+ is normally about 100:1 in liver cytosol. -

Improving the Utilization of Isomaltose and Panose by Lager Yeast Saccharomyces Pastorianus
fermentation Article Improving the Utilization of Isomaltose and Panose by Lager Yeast Saccharomyces pastorianus Javier Porcayo Loza 1,2,† , Anna Chailyan 3, Jochen Forster 3 , Michael Katz 3, Uffe Hasbro Mortensen 2,* and Rosa Garcia Sanchez 3,* 1 Novo Nordisk Foundation Center for Biosustainability, Technical University of Denmark, 2800 Kongens Lyngby, Denmark; [email protected] 2 Department of Bioengineering, Technical University of Denmark, 2800 Kongens Lyngby, Denmark 3 Carlsberg A/S, Carlsberg Research Laboratory, 1799 Copenhagen V, Denmark; [email protected] (A.C.); [email protected] (J.F.); [email protected] (M.K.) * Correspondence: [email protected] (U.H.M.); [email protected] (R.G.S.) † Current address: Graphenea S.A., 20009 San Sebastian, Spain. Abstract: Approximately 25% of all carbohydrates in industrial worts are poorly, if at all, fermented by brewing yeast. This includes dextrins, β-glucans, arabinose, xylose, disaccharides such as isomaltose, nigerose, kojibiose, and trisaccharides such as panose and isopanose. As the efficient utilization of carbohydrates during the wort’s fermentation impacts the alcohol yield and the organoleptic traits of the product, developing brewing strains with enhanced abilities to ferment subsets of these sugars is highly desirable. In this study, we developed Saccharomyces pastorianus laboratory yeast strains with a superior capacity to grow on isomaltose and panose. First, we designed a plasmid toolbox for Citation: Porcayo Loza, J.; Chailyan, the stable integration of genes into lager strains. Next, we used the toolbox to elevate the levels of A.; Forster, J.; Katz, M.; Mortensen, the α-glucoside transporter Agt1 and the major isomaltase Ima1. -

Biochemistry Entry of Fructose and Galactose
Paper : 04 Metabolism of carbohydrates Module : 06 Entry of Fructose and Galactose Dr. Vijaya Khader Dr. MC Varadaraj Principal Investigator Dr.S.K.Khare,Professor IIT Delhi. Paper Coordinator Dr. Ramesh Kothari,Professor UGC-CAS Department of Biosciences Saurashtra University, Rajkot-5, Gujarat-INDIA Dr. S. P. Singh, Professor Content Reviewer UGC-CAS Department of Biosciences Saurashtra University, Rajkot-5, Gujarat-INDIA Dr. Charmy Kothari, Assistant Professor Content Writer Department of Biotechnology Christ College, Affiliated to Saurashtra University, Rajkot-5, Gujarat-INDIA 1 Metabolism of Carbohydrates Biochemistry Entry of Fructose and Galactose Description of Module Subject Name Biochemistry Paper Name 04 Metabolism of Carbohydrates Module Name/Title 06 Entry of Fructose and Galactose 2 Metabolism of Carbohydrates Biochemistry Entry of Fructose and Galactose METABOLISM OF FRUCTOSE Objectives 1. To study the major pathway of fructose metabolism 2. To study specialized pathways of fructose metabolism 3. To study metabolism of galactose 4. To study disorders of galactose metabolism 3 Metabolism of Carbohydrates Biochemistry Entry of Fructose and Galactose Introduction Sucrose disaccharide contains glucose and fructose as monomers. Sucrose can be utilized as a major source of energy. Sucrose includes sugar beets, sugar cane, sorghum, maple sugar pineapple, ripe fruits and honey Corn syrup is recognized as high fructose corn syrup which gives the impression that it is very rich in fructose content but the difference between the fructose content in sucrose and high fructose corn syrup is only 5-10%. HFCS is rich in fructose because the sucrose extracted from the corn syrup is treated with the enzyme that converts some glucose in fructose which makes it more sweet. -

The Chemical Isomerization of Lactose to Lactulose by Using Sodium Hydroxide As Batch Reaction
Pak. J. Chem. 5(3): 24-30, 2016 Full Paper ISSN (Print): 2220-2625 ISSN (Online): 2222-307X DOI: 10.15228/2016.v06.i01-2.p04 The Chemical Isomerization of Lactose to Lactulose by Using Sodium Hydroxide as Batch Reaction *Nahla T. K. Department of Food Sciences, College of Agriculture, University of Baghdad, Iraq E-mail: *[email protected] ABSTRACT Lactulose is a synthetic and non-absorbable disaccharide sugar containing glucose and fructose units. It is used for the treatment of constipation and hepatic encephalopathy. The purpose of this experiment was to synthesized lactulose in the presence of sodium hydroxide as a catalyst. The evaluation of lactulose synthesis was carried out at different concentration of sodium hydroxide (1, 2 and 3M) and the study was also conducted at different temperatures (30, 50, 70, and 90 °C). In addition, the kinetics of lactose isomerization with respect to the time also studied. Finally, it was concluded that the lactulose formation was determined by spectrophotometric method. The lactulose isomerization increased about more than 30.9% at 90 °C in the presence of 1M sodium hydroxide and 20 % lactose concentration. Keywords: Lactose, isomerization, lactulose, sodium hydroxide. 1. INTRODUCTION Lactulose is a synthetic sugar, which is not adsorbed by humans or rodents due to the lack of enzymes which catalyzes the disaccharide sugars. It can be generated by either alkaline isomerization of lactose via the Lobry de Bruyn - Alberda van Ekenstein rearrangement or by enzyme-catalyzed synthesis. Based on the first reaction, different process schemes for the preparation of lactulose have been developed. The enzymatic synthesis of lactulose can be carried out using different pathways with the transgalactosylation reaction being the most promising1. -

LACTOSE & D-GALACTOSE (Rapid)
www.megazyme.com LACTOSE & D-GALACTOSE (Rapid) ASSAY PROCEDURE K-LACGAR 02/21 Incorporating A Procedure For The Analysis Of “Low- Lactose” Or “Lactose-Free” Samples Containing High Levels Of Monosaccharides (Improved Rapid Format) (*115 Assays per Kit) * The number of tests per kit can be doubled if all volumes are halved The reagents provided in this kit are also suitable for use with AOAC method 2006.06 – Lactose in milk. Patented: US 7,785,771 B2 and EP1 828 407 (GB, FR, IE, DE) © Megazyme 2021 INTRODUCTION: Lactose, or milk sugar, is a white crystalline disaccharide. It is formed in the mammary glands of all lactating animals and is present in their milk. Lactose yields D-galactose and D-glucose on hydrolysis by lactase (β-galactosidase), an enzyme found in gastric juice. People who lack this enzyme after childhood cannot digest milk and are said to be lactose intolerant. Common symptoms of lactose intolerance include nausea, cramps, gas and diarrhoea, which begin about 30 minutes to 2 hours after eating or drinking foods containing lactose. Between 30 and 50 million Americans are lactose intolerant, with certain ethnic and racial populations being more widely affected than others; as many as 75 percent of all African-Americans and Native Americans and 90 percent of Asian-Americans are lactose intolerant. The condition is least common among persons of northern European descent. Enzymic methods for the measurement of lactose are well known and are generally based on the hydrolysis of lactose to D-galactose and D-glucose with β-galactosidase, followed by determination of either D-galactose or D-glucose. -

Congenital Sucrase-Isomaltase Deficiency
Congenital sucrase-isomaltase deficiency Description Congenital sucrase-isomaltase deficiency is a disorder that affects a person's ability to digest certain sugars. People with this condition cannot break down the sugars sucrose and maltose. Sucrose (a sugar found in fruits, and also known as table sugar) and maltose (the sugar found in grains) are called disaccharides because they are made of two simple sugars. Disaccharides are broken down into simple sugars during digestion. Sucrose is broken down into glucose and another simple sugar called fructose, and maltose is broken down into two glucose molecules. People with congenital sucrase- isomaltase deficiency cannot break down the sugars sucrose and maltose, and other compounds made from these sugar molecules (carbohydrates). Congenital sucrase-isomaltase deficiency usually becomes apparent after an infant is weaned and starts to consume fruits, juices, and grains. After ingestion of sucrose or maltose, an affected child will typically experience stomach cramps, bloating, excess gas production, and diarrhea. These digestive problems can lead to failure to gain weight and grow at the expected rate (failure to thrive) and malnutrition. Most affected children are better able to tolerate sucrose and maltose as they get older. Frequency The prevalence of congenital sucrase-isomaltase deficiency is estimated to be 1 in 5, 000 people of European descent. This condition is much more prevalent in the native populations of Greenland, Alaska, and Canada, where as many as 1 in 20 people may be affected. Causes Mutations in the SI gene cause congenital sucrase-isomaltase deficiency. The SI gene provides instructions for producing the enzyme sucrase-isomaltase. -

Concentration and Distribution of Sialic Acid in Human Milk and Infant Formulas1–3
Concentration and distribution of sialic acid in human milk and infant formulas1–3 Bing Wang, Janette Brand-Miller, Patricia McVeagh, and Peter Petocz Downloaded from https://academic.oup.com/ajcn/article/74/4/510/4737459 by guest on 23 September 2021 ABSTRACT 2- to 4-fold higher than those of other mammals, including chim- Background: In animal studies, sialic acid supplementation is panzees (4). Sialic acid is thought to play a role in the structural associated with increases of gangliosides in the brain and and functional establishment of synaptic pathways: >40% of improved learning ability. Only limited data are available on the sialic acid in the brain is found in the synaptosomal fraction and sialic acid content of human milk and infant formulas. contributes to the negative charge of the membrane (3). Because Objective: We compared the concentrations of oligosaccharide- most neurotransmitters are positively charged, sialic acid may bound, protein-bound, and free sialic acid in milk from mothers of assist neurotransmission by facilitating the binding of transmit- full-term and preterm infants and in a range of infant formulas. ter molecules to the synaptic membrane. Design: The milk from 20 and 14 mothers of full-term and Morgan and Winick (2) showed that exogenous sialic acid preterm infants (mean gestational age: 31 ± 3 wk), respectively, administered by intraperitoneal injection increased the produc- was collected at 4 stages of lactation (colostrum, transition, 1 mo, tion of ganglioside sialic acid in the brain and improved learning and 3 mo) and compared with 21 different infant formulas. ability in well-nourished and malnourished rat pups. -

Structural Features
1 Structural features As defined by the International Union of Pure and Applied Chemistry gly- cans are structures of multiple monosaccharides linked through glycosidic bonds. The terms sugar and saccharide are synonyms, depending on your preference for Arabic (“sukkar”) or Greek (“sakkēaron”). Saccharide is the root for monosaccha- rides (a single carbohydrate unit), oligosaccharides (3 to 20 units) and polysac- charides (large polymers of more than 20 units). Carbohydrates follow the basic formula (CH2O)N>2. Glycolaldehyde (CH2O)2 would be the simplest member of the family if molecules of two C-atoms were not excluded from the biochemical repertoire. Glycolaldehyde has been found in space in cosmic dust surrounding star-forming regions of the Milky Way galaxy. Glycolaldehyde is a precursor of several organic molecules. For example, reaction of glycolaldehyde with propenal, another interstellar molecule, yields ribose, a carbohydrate that is also the backbone of nucleic acids. Figure 1 – The Rho Ophiuchi star-forming region is shown in infrared light as captured by NASA’s Wide-field Infrared Explorer. Glycolaldehyde was identified in the gas surrounding the star-forming region IRAS 16293-2422, which is is the red object in the centre of the marked square. This star-forming region is 26’000 light-years away from Earth. Glycolaldehyde can react with propenal to form ribose. Image source: www.eso.org/public/images/eso1234a/ Beginning the count at three carbon atoms, glyceraldehyde and dihydroxy- acetone share the common chemical formula (CH2O)3 and represent the smallest carbohydrates. As their names imply, glyceraldehyde has an aldehyde group (at C1) and dihydoxyacetone a carbonyl group (at C2). -

Xorox Univerelty Microfilms
INFORMATION TO USERS This material was producad from a microfilm copy of the original document. While the moit advanced technological meant to photograph and reproduce thii document have been used, the quality it heavily dependent upon the quality of the original submitted. The following explanation of techniques is provided to help ou understand markings or patterns which may appear on this reproduction. 1. The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to ob tain the mining page(s) or section, they are spliced into the film along w ith: adjacent pages, This may have necessitated cutting thru an image and dupli eating adjacent pages to insure you complete continuity. 2. When an image on the film is obliterated with a large round black mark, it is an indication that the photographer suspected that the copy may have moved during exposure and thus cause a blurred image, fou will find a good image of the page in the adjacent frame. 3. When a map, drawing or chart, etc., was part of the material being photographed the photographer followed a definite method in "sectioning” the material. It is customary to begin photoi ig at the upper left hand comer of a large dieet and to continue photoi ig from left to right in equal sections with a small overlap. If necessary, sectioning is continued agein — beginning below the first row and continuing on until complete. 4. The majority of users indicate that the textual content is of greatest value, however, a somewhat higher quality reproduction could be made from "photographs" if essential to the understanding of the dissertation. -

Lactulose Solution, Usp
LACTULOSE- lactulose solution VistaPharm, Inc ---------- LACTULOSE SOLUTION, USP Rx Only VP2051 04/10 DESCRIPTION Lactulose is a synthetic disaccharide in solution form for oral administration. Each 15 mL of lactulose solution contains: 10 g lactulose (and less than 1.6 g galactose, less than 1.2 g lactose, and 1.2 g of other sugars). Also contains FD&C Yellow No. 6, purified water, USP and wild cherry flavoring. A minimal quantity of sodium hydroxide, NF is used to adjust pH when necessary. The pH range is 2.5 to 6.5. Lactulose is a colonic acidifier which promotes laxation.The chemical name for lactulose is 4-O-β-D- galactopyranosyl-D-fructofuranose. It has the following structural formula: C12H22O11 The molecular weight is 342.30. It is freely soluble in water. CLINICAL PHARMACOLOGY Lactulose is poorly absorbed from the gastrointestinal tract and no enzyme capable of hydrolysis of this disaccharide is present in human gastrointestinal tissue. As a result, oral doses of lactulose reach the colon virtually unchanged. In the colon, lactulose is broken down primarily to lactic acid, and also to small amounts of formic and acetic acids, by the action of colonic bacteria, which results in an increase in osmotic pressure and slight acidification of the colonic contents. This in turn causes an increase in stool water content and softens the stool. Since lactulose does not exert its effect until it reaches the colon, and since transit time through the colon may be slow, 24 to 48 hours may be required to produce the desired bowel movement. -
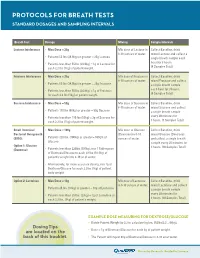
Protocols for Breath Tests Interpretation Help Standard Dosages and Sampling Intervals for Hydrogen/Methane Breath Tests
PROTOCOLS FOR BREATH TESTS INTERPRETATION HELP STANDARD DOSAGES AND SAMPLING INTERVALS FOR HYDROGEN/METHANE BREATH TESTS Breath Test Dosage Mixing Sample Intervals LACTOSE, FRUCTOSE OR SUCROSE INTOLERANCE: Lactose Intolerance • Max Dose = 25g Mix dose of Lactose in Collect Baseline, drink SUSPECTED POSITIVE: 8-10 ounces of water. mixed Lactose and collect a • Hydrogen (H ) Production Only: 20 ppm delta increase in Hydrogen from the lowest preceding value in the test. • Patients 55 lbs (24.9kg) or greater = 25g Lactose single breath sample each 2 hour for 3 hours. • Patients less than 55 lbs (24.9kg) = 1g of Lactose for • Methane (CH4) Production Only: 12 ppm delta increase in Methane from the lowest preceding value in the test. (4 Samples Total) each 2.2 lbs (1kg) of patient weight. • Hydrogen/Methane Both Produced: Add both H2 and CH4 values together in each sample. Review for a 15 ppm delta increase from the lowest preceding value. Fructose Intolerance • Max Dose = 25g Mix dose of Fructose in Collect Baseline, drink 8-10 ounces of water. mixed Fructose and collect • Patients 55 lbs (24.9kg) or greater = 25g Fructose a single breath sample each hour for 3 hours. SMALL INTESTINAL BACTERIAL OVERGROWTH (SIBO): • Patients less than 55 lbs (24.9kg) = 1g of Fructose (4 Samples Total) for each 2.2 lbs (1kg) of patient weight. SUSPECTED POSITIVE: Sucrose Intolerance • Max Dose = 50g Mix dose of Sucrose in Collect Baseline, drink OPTION 1: GLUCOSE (DEXTROSE) 8-10 ounces of water. mixed Sucrose and collect • Patients 110 lbs (50kg) or greater = 50g Sucrose • Hydrogen (H ) Production Only: 12 ppm delta increase in a single breath sample 2 every 30 minutes for Hydrogen from the lowest preceding value in the test. -

Glucose-Galactose Malabsorption J
Arch Dis Child: first published as 10.1136/adc.42.226.592 on 1 December 1967. Downloaded from Arch. Dis. Childh., 1967, 42, 592. Glucose-galactose Malabsorption J. M. ABRAHAM, B. LEVIN, V. G. OBERHOLZER, and ALEX RUSSELL* From Queen Elizabeth Hospitalfor Children, Hackney Road, London E.2 Primary disaccharidase deficiency is a well-known electrolytaemia. On the 16th day, therefore, feeds cause of diarrhoea in infancy. The offending were changed to Nutramigen without improvement, and disaccharide appears in the stool after ingestion due 9 days later this was substituted by Velactin. Both of to the absence in the intestinal mucosa of the appro- these low-lactose preparations contain, in addition to protein, large amounts of dextrin and starch, and priate splitting enzyme. A much rarer cause of Nutramigen also includes maltose, while Velactin also has diarrhoea in this period is a failure of absorption of glucose and sucrose. All these carbohydrates on the monosaccharides, glucose and galactose, in the digestion give rise to glucose. The diarrhoea now presence of histologically normal mucosa and full decreased and the stools became less liquid; but disaccharidase activity. The clinical and bio- there was no weight gain (Fig. 1) and glucose was still chemical picture of this newly-described entity is present in the stools. After 12 days on this regime, an characteristic. Watery diarrhoea, with glucose extensive rash developed on the buttocks and groins, and/or galactose in the stools, develops within three with stomatitis (Fig. 2), anaemia, hypokalaemia, and days of milk feeding and continues as long as the hyperchloraemic acidosis (Na 138, K 2-4, Cl 112, standard bicarbonate 13*7 mEq/l.).