Xorox Univerelty Microfilms
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Liquid Chromatography with Electrospray Ionization And
Hindawi Publishing Corporation International Journal of Analytical Chemistry Volume 2016, Article ID 9269357, 8 pages http://dx.doi.org/10.1155/2016/9269357 Research Article Liquid Chromatography with Electrospray Ionization and Tandem Mass Spectrometry Applied in the Quantitative Analysis of Chitin-Derived Glucosamine for a Rapid Estimation of Fungal Biomass in Soil Madelen A. Olofsson and Dan Bylund DepartmentofNaturalSciences,MidSwedenUniversity,85170Sundsvall,Sweden Correspondence should be addressed to Madelen A. Olofsson; [email protected] Received 2 September 2015; Accepted 12 January 2016 Academic Editor: Frantisek Foret Copyright © 2016 M. A. Olofsson and D. Bylund. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. This method employs liquid chromatography-tandem mass spectrometry to rapidly quantify chitin-derived glucosamine for estimating fungal biomass. Analyte retention was achieved using hydrophilic interaction liquid chromatography, with a zwitter- ionic stationary phase (ZIC-HILIC), and isocratic elution using 60% 5 mM ammonium formate buffer (pH 3.0) and 40% ACN. Inclusion of muramic acid and its chromatographic separation from glucosamine enabled calculation of the bacterial contribution to the latter. Galactosamine, an isobaric isomer to glucosamine, found in significant amounts in soil samples, was also investigated. Thetwoisomersformthesameprecursorandproductionsandcouldnotbechromatographicallyseparatedusingthisrapid method. Instead, glucosamine and galactosamine were distinguished mathematically, using the linear relationships describing the differences in product ion intensities for the two analytes. The m/z transitions of 180 → 72 and 180 → 84 were applied for the detection of glucosamine and galactosamine and that of 252 → 126 for muramic acid. -

Chapter 12 Slides
11/15/17 CHAPTER 12: Carbohydrates: Structure and Function OUTLINE • 12.1 Role of Carbohydrates • 12.2 Monosaccharides • 12.3 Complex Carbohydrates • 12.4 Carbohydrate Catabolism • 12.5 Oligosaccharides as Cell Markers CHAPTER 12: Carbohydrates: Structure and Function WHAT ARE CARBOHYDRATES? • Glucose and its derivatives are carbohydrates: Ø Carbohydrates are simple organic molecules that have a shared basic chemical Formula: Cn(H2O)n Ø The name “carbo + hydrate” represents that Fact that they are made from CO2 and H2O by photosynthesis • About halF oF all earth’s solid carbon is Found in two polymers of glucose found in plants: Ø Starch = major energy storage molecule Ø Cellulose = major structural component oF the plant cell wall (aka. “fiber”) CHAPTER 12: Carbohydrates: Structure and Function THE SIMPLEST CARBOHYDRATES • Monosaccharides are carbohydrates that cannot be hydrolyZed into simpler carbohydrates: Ø These are the Fundamental building blocks For all other carbohydrates (oFten called “simple sugars”) Ø All have Formulas of based on the basic pattern: Cn(H2O)n • Monosaccharides have speciFic Functional groups: 1. An aldehyde OR a ketone (not both!) 2. Several (two or more) alcohol (-OH) groups 1 11/15/17 CHAPTER 12: Carbohydrates: Structure and Function STRUCTURE & NOMENCLATURE OF MONOSACCHARIDES • Monosaccharides are classiFied by two features: 1. Length of their main carbon chain (utilize standard IUPAC naming For # oF carbons) 2. Whether they contain an aldehyde or ketone group • Names always end with –ose • Two common hexoses: -

Amino Sugars and Muramic Acid—Biomarkers for Soil Microbial Community Structure Analysis
Soil Biology & Biochemistry 36 (2004) 399–407 www.elsevier.com/locate/soilbio Amino sugars and muramic acid—biomarkers for soil microbial community structure analysis Bruno Glasera,*, Marı´a-Bele´n Turrio´nb, Kassem Alefc aInstitute of Soil Science and Soil Geography, University of Bayreuth, Bayreuth D-95440, Germany bArea of Soil Science and Soil Chemistry, ETSIIAA, University of Valladolid, Palencia 34004, Spain cUmwelt und Technologie Consulting, Bayreuth D-95448, Germany Received 13 December 2002; received in revised form 22 July 2003; accepted 7 October 2003 Abstract Characterizing functional and phylogenetic microbial community structure in soil is important for understanding the fate of microbially- derived compounds during the decomposition and turn-over of soil organic matter. This study was conducted to test whether amino sugars and muramic acid are suitable biomarkers to trace bacterial, fungal, and actinomycetal residues in soil. For this aim, we investigated the pattern, amounts, and dynamics of three amino sugars (glucosamine, mannosamine and galactosamine) and muramic acid in the total microbial biomass and selectively cultivated bacteria, fungi, and actinomycetes offive different soils amended with and without glucose. Our results revealed that total amino sugar and muramic acid concentrations in microbial biomass, extracted from soil after chloroform fumigation varied between 1 and 27 mg kg21 soil. In all soils investigated, glucose addition resulted in a 50–360% increase of these values. In reference to soil microbial biomass-C, the total amino sugar- and muramic acid-C concentrations ranged from 1–71 g C kg21 biomass-C. After an initial lag phase, the cultivated microbes revealed similar amino sugar concentrations of about 35, 27 and 17 g glucosamine-C kg21 TOC in bacteria, fungi, and actinomycetes, respectively. -

The Chemical Isomerization of Lactose to Lactulose by Using Sodium Hydroxide As Batch Reaction
Pak. J. Chem. 5(3): 24-30, 2016 Full Paper ISSN (Print): 2220-2625 ISSN (Online): 2222-307X DOI: 10.15228/2016.v06.i01-2.p04 The Chemical Isomerization of Lactose to Lactulose by Using Sodium Hydroxide as Batch Reaction *Nahla T. K. Department of Food Sciences, College of Agriculture, University of Baghdad, Iraq E-mail: *[email protected] ABSTRACT Lactulose is a synthetic and non-absorbable disaccharide sugar containing glucose and fructose units. It is used for the treatment of constipation and hepatic encephalopathy. The purpose of this experiment was to synthesized lactulose in the presence of sodium hydroxide as a catalyst. The evaluation of lactulose synthesis was carried out at different concentration of sodium hydroxide (1, 2 and 3M) and the study was also conducted at different temperatures (30, 50, 70, and 90 °C). In addition, the kinetics of lactose isomerization with respect to the time also studied. Finally, it was concluded that the lactulose formation was determined by spectrophotometric method. The lactulose isomerization increased about more than 30.9% at 90 °C in the presence of 1M sodium hydroxide and 20 % lactose concentration. Keywords: Lactose, isomerization, lactulose, sodium hydroxide. 1. INTRODUCTION Lactulose is a synthetic sugar, which is not adsorbed by humans or rodents due to the lack of enzymes which catalyzes the disaccharide sugars. It can be generated by either alkaline isomerization of lactose via the Lobry de Bruyn - Alberda van Ekenstein rearrangement or by enzyme-catalyzed synthesis. Based on the first reaction, different process schemes for the preparation of lactulose have been developed. The enzymatic synthesis of lactulose can be carried out using different pathways with the transgalactosylation reaction being the most promising1. -

Concentration and Distribution of Sialic Acid in Human Milk and Infant Formulas1–3
Concentration and distribution of sialic acid in human milk and infant formulas1–3 Bing Wang, Janette Brand-Miller, Patricia McVeagh, and Peter Petocz Downloaded from https://academic.oup.com/ajcn/article/74/4/510/4737459 by guest on 23 September 2021 ABSTRACT 2- to 4-fold higher than those of other mammals, including chim- Background: In animal studies, sialic acid supplementation is panzees (4). Sialic acid is thought to play a role in the structural associated with increases of gangliosides in the brain and and functional establishment of synaptic pathways: >40% of improved learning ability. Only limited data are available on the sialic acid in the brain is found in the synaptosomal fraction and sialic acid content of human milk and infant formulas. contributes to the negative charge of the membrane (3). Because Objective: We compared the concentrations of oligosaccharide- most neurotransmitters are positively charged, sialic acid may bound, protein-bound, and free sialic acid in milk from mothers of assist neurotransmission by facilitating the binding of transmit- full-term and preterm infants and in a range of infant formulas. ter molecules to the synaptic membrane. Design: The milk from 20 and 14 mothers of full-term and Morgan and Winick (2) showed that exogenous sialic acid preterm infants (mean gestational age: 31 ± 3 wk), respectively, administered by intraperitoneal injection increased the produc- was collected at 4 stages of lactation (colostrum, transition, 1 mo, tion of ganglioside sialic acid in the brain and improved learning and 3 mo) and compared with 21 different infant formulas. ability in well-nourished and malnourished rat pups. -

Supplementary Data
Metabolomics in cancer research: Supplementary data 1 Supplementary Data 2 Supplementary methods 3 Additional information regarding the search strategy used in the main paper 4 Supplementary tables 5 Supplementary Table 1: List of reported metabolites to be altered in human cancers 6 Supplementary figures 7 Supplementary Figure 1: General overview over the metabolomics workflow 8 1 Metabolomics in cancer research: Supplementary data 9 Supplementary methods 10 Search strategy 11 The search strategy was described in the main paper. For the Web of Knowledge search an 12 additional refinement step was necessary to reduce irrelevant findings. The following research 13 areas of low relevance were excluded: 14 Plant Science, Biophysics, Agriculture, Environmental Sciences Ecology, Microbiology, 15 Computer Science, Mathematics, Cardiology, Engineering, Automation control Systems, 16 Marine Freshwater Biology, Behavioral Science, Physics, Developmental Biology, Zoology, 17 Psychiatry, Energy Fuels, Infectious Disease, Parasitology, Mycology, Rheumatology, 18 Psychology, Veterinary Sciences, Dentistry, Legal Medicine, Polymer Science, 19 Anesthesiology, Robotics, Sports Science, Forestry, Tropical Medicine, Virology, Water 20 Resources, Electrochemistry, Evolutionary Biology, Fisheries, Materials Science, 21 Oceanography, Substance Abuse, Geology, Nuclear Science Technology, Operations 22 Research Management, Orthopedics, Allergy, Biodiversity, Educational Research, 23 Metallurgy, Optics, Emergency Medicine, Geochemistry, History philosophy of science, 24 Mathematical methods in Social sciences, Mechanics, Social Issues, Thermodynamics 25 Additionally the abstracts had to contain one of the following keywords: 26 “MS” OR “mass spectrometry” OR “patients”. 2 Metabolomics in cancer research: Supplementary data 27 Supplementary tables 28 Supplementary Table 1: List of reported metabolites altered in human cancers. Metabolites in bold were reported from a study that validated 29 findings within an independent study population. -

Lactulose Solution, Usp
LACTULOSE- lactulose solution VistaPharm, Inc ---------- LACTULOSE SOLUTION, USP Rx Only VP2051 04/10 DESCRIPTION Lactulose is a synthetic disaccharide in solution form for oral administration. Each 15 mL of lactulose solution contains: 10 g lactulose (and less than 1.6 g galactose, less than 1.2 g lactose, and 1.2 g of other sugars). Also contains FD&C Yellow No. 6, purified water, USP and wild cherry flavoring. A minimal quantity of sodium hydroxide, NF is used to adjust pH when necessary. The pH range is 2.5 to 6.5. Lactulose is a colonic acidifier which promotes laxation.The chemical name for lactulose is 4-O-β-D- galactopyranosyl-D-fructofuranose. It has the following structural formula: C12H22O11 The molecular weight is 342.30. It is freely soluble in water. CLINICAL PHARMACOLOGY Lactulose is poorly absorbed from the gastrointestinal tract and no enzyme capable of hydrolysis of this disaccharide is present in human gastrointestinal tissue. As a result, oral doses of lactulose reach the colon virtually unchanged. In the colon, lactulose is broken down primarily to lactic acid, and also to small amounts of formic and acetic acids, by the action of colonic bacteria, which results in an increase in osmotic pressure and slight acidification of the colonic contents. This in turn causes an increase in stool water content and softens the stool. Since lactulose does not exert its effect until it reaches the colon, and since transit time through the colon may be slow, 24 to 48 hours may be required to produce the desired bowel movement. -
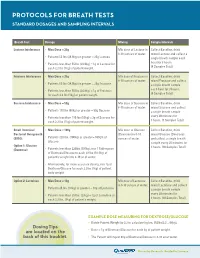
Protocols for Breath Tests Interpretation Help Standard Dosages and Sampling Intervals for Hydrogen/Methane Breath Tests
PROTOCOLS FOR BREATH TESTS INTERPRETATION HELP STANDARD DOSAGES AND SAMPLING INTERVALS FOR HYDROGEN/METHANE BREATH TESTS Breath Test Dosage Mixing Sample Intervals LACTOSE, FRUCTOSE OR SUCROSE INTOLERANCE: Lactose Intolerance • Max Dose = 25g Mix dose of Lactose in Collect Baseline, drink SUSPECTED POSITIVE: 8-10 ounces of water. mixed Lactose and collect a • Hydrogen (H ) Production Only: 20 ppm delta increase in Hydrogen from the lowest preceding value in the test. • Patients 55 lbs (24.9kg) or greater = 25g Lactose single breath sample each 2 hour for 3 hours. • Patients less than 55 lbs (24.9kg) = 1g of Lactose for • Methane (CH4) Production Only: 12 ppm delta increase in Methane from the lowest preceding value in the test. (4 Samples Total) each 2.2 lbs (1kg) of patient weight. • Hydrogen/Methane Both Produced: Add both H2 and CH4 values together in each sample. Review for a 15 ppm delta increase from the lowest preceding value. Fructose Intolerance • Max Dose = 25g Mix dose of Fructose in Collect Baseline, drink 8-10 ounces of water. mixed Fructose and collect • Patients 55 lbs (24.9kg) or greater = 25g Fructose a single breath sample each hour for 3 hours. SMALL INTESTINAL BACTERIAL OVERGROWTH (SIBO): • Patients less than 55 lbs (24.9kg) = 1g of Fructose (4 Samples Total) for each 2.2 lbs (1kg) of patient weight. SUSPECTED POSITIVE: Sucrose Intolerance • Max Dose = 50g Mix dose of Sucrose in Collect Baseline, drink OPTION 1: GLUCOSE (DEXTROSE) 8-10 ounces of water. mixed Sucrose and collect • Patients 110 lbs (50kg) or greater = 50g Sucrose • Hydrogen (H ) Production Only: 12 ppm delta increase in a single breath sample 2 every 30 minutes for Hydrogen from the lowest preceding value in the test. -

REVIEW the Role and Potential of Sialic Acid in Human Nutrition
European Journal of Clinical Nutrition (2003) 57, 1351–1369 & 2003 Nature Publishing Group All rights reserved 0954-3007/03 $25.00 www.nature.com/ejcn REVIEW The role and potential of sialic acid in human nutrition B Wang1* and J Brand-Miller1 1Human Nutrition Unit, School of Molecular and Microbial Biosciences, University of Sydney, NSW, Australia Sialic acids are a family of nine-carbon acidic monosaccharides that occur naturally at the end of sugar chains attached to the surfaces of cells and soluble proteins. In the human body, the highest concentration of sialic acid (as N-acetylneuraminic acid) occurs in the brain where it participates as an integral part of ganglioside structure in synaptogenesis and neural transmission. Human milk also contains a high concentration of sialic acid attached to the terminal end of free oligosaccharides, but its metabolic fate and biological role are currently unknown. An important question is whether the sialic acid in human milk is a conditional nutrient and confers developmental advantages on breast-fed infants compared to those fed infant formula. In this review, we critically discuss the current state of knowledge of the biology and role of sialic acid in human milk and nervous tissue, and the link between sialic acid, breastfeeding and learning behaviour. European Journal of Clinical Nutrition (2003) 57, 1351–1369. doi:10.1038/sj.ejcn.1601704 Keywords: sialic acid; ganglioside; sialyl-oligosaccharides; human milk; infant formula; breastfeeding Introduction promising new candidate is sialic acid (also known as The rapid growth and development of the newborn infant N-acetylneuraminic acid), a nine-carbon sugar that is a puts exceptional demands on the supply of nutrients. -

Analysis of Proteomic Responses of Freeze-Dried Oenococcus Oeni to Access the Molecular Mechanism of Acid Acclimation on Cell Freeze-Drying Resistance
Accepted Manuscript Analysis of proteomic responses of freeze-dried Oenococcus oeni to access the molecular mechanism of acid acclimation on cell freeze-drying resistance Kun Yang, Yang Zhu, Yiman Qi, Tingjing Zhang, Miaomiao Liu, Jie Zhang, Xinyuan Wei, Mingtao Fan, Guoqiang Zhang PII: S0308-8146(19)30188-8 DOI: https://doi.org/10.1016/j.foodchem.2019.01.120 Reference: FOCH 24215 To appear in: Food Chemistry Received Date: 2 October 2018 Revised Date: 24 December 2018 Accepted Date: 17 January 2019 Please cite this article as: Yang, K., Zhu, Y., Qi, Y., Zhang, T., Liu, M., Zhang, J., Wei, X., Fan, M., Zhang, G., Analysis of proteomic responses of freeze-dried Oenococcus oeni to access the molecular mechanism of acid acclimation on cell freeze-drying resistance, Food Chemistry (2019), doi: https://doi.org/10.1016/j.foodchem. 2019.01.120 This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain. Analysis of proteomic responses of freeze-dried Oenococcus oeni to access the molecular mechanism of acid acclimation on cell freeze-drying resistance Kun Yang1,2, Yang Zhu3, Yiman Qi2,Tingjing Zhang4, Miaomiao Liu2, Jie Zhang2, Xinyuan Wei2, Mingtao Fan2,*, Guoqiang Zhang1,* 1 College of Biological and Chemical Engineering, Anhui Polytechnic University, Wuhu, 241000, China 2 College of Food Science and Engineering, Northwest A & F University, Yangling, 712100, China 3 School of Agriculture and Food Sciences, University of Queensland, QLD, 4046, Australia 4 College of Food Science and Technology, Henan University of Technology, Zhenzhou, 450001, China * Corresponding author: 1. -

The Metabolic Building Blocks of a Minimal Cell Supplementary
The metabolic building blocks of a minimal cell Mariana Reyes-Prieto, Rosario Gil, Mercè Llabrés, Pere Palmer and Andrés Moya Supplementary material. Table S1. List of enzymes and reactions modified from Gabaldon et. al. (2007). n.i.: non identified. E.C. Name Reaction Gil et. al. 2004 Glass et. al. 2006 number 2.7.1.69 phosphotransferase system glc + pep → g6p + pyr PTS MG041, 069, 429 5.3.1.9 glucose-6-phosphate isomerase g6p ↔ f6p PGI MG111 2.7.1.11 6-phosphofructokinase f6p + atp → fbp + adp PFK MG215 4.1.2.13 fructose-1,6-bisphosphate aldolase fbp ↔ gdp + dhp FBA MG023 5.3.1.1 triose-phosphate isomerase gdp ↔ dhp TPI MG431 glyceraldehyde-3-phosphate gdp + nad + p ↔ bpg + 1.2.1.12 GAP MG301 dehydrogenase nadh 2.7.2.3 phosphoglycerate kinase bpg + adp ↔ 3pg + atp PGK MG300 5.4.2.1 phosphoglycerate mutase 3pg ↔ 2pg GPM MG430 4.2.1.11 enolase 2pg ↔ pep ENO MG407 2.7.1.40 pyruvate kinase pep + adp → pyr + atp PYK MG216 1.1.1.27 lactate dehydrogenase pyr + nadh ↔ lac + nad LDH MG460 1.1.1.94 sn-glycerol-3-phosphate dehydrogenase dhp + nadh → g3p + nad GPS n.i. 2.3.1.15 sn-glycerol-3-phosphate acyltransferase g3p + pal → mag PLSb n.i. 2.3.1.51 1-acyl-sn-glycerol-3-phosphate mag + pal → dag PLSc MG212 acyltransferase 2.7.7.41 phosphatidate cytidyltransferase dag + ctp → cdp-dag + pp CDS MG437 cdp-dag + ser → pser + 2.7.8.8 phosphatidylserine synthase PSS n.i. cmp 4.1.1.65 phosphatidylserine decarboxylase pser → peta PSD n.i. -

LACTULOSE SOLUTION USP - Lactulose Solution Hi-Tech Pharmacal Co., Inc
LACTULOSE SOLUTION USP - lactulose solution Hi-Tech Pharmacal Co., Inc. DESCRIPTION Lactulose is a synthetic disaccharide in solution form for oral administration. Each 15 mL of lactulose solution contains: 10 g lactulose (and less than 1.6 g galactose, less than 1.2 g lactose, and 1.2 g or less of other sugars). Lactulose solution contains potassium sorbate as an inactive ingredient. Lactulose is a colonic acidifier which promotes laxation. The chemical name for lactulose is 4-O-β-D-galactopyranosyl-D-fructofuranose. It has the following structural formula: The molecular weight is 342.30. It is freely soluble in water. CLINICAL PHARMACOLOGY Lactulose is poorly absorbed from the gastrointestinal tract and no enzyme capable of hydrolysis of this disaccharide is present in human gastrointestinal tissue. As a result, oral doses of lactulose reach the colon virtually unchanged. In the colon, lactulose is broken down primarily to lactic acid, and also to small amounts of formic and acetic acids, by the action of colonic bacteria, which results in an increase in osmotic pressure and slight acidification of the colonic contents. This in turn causes an increase in stool water content and softens the stool. Since lactulose does not exert its effect until it reaches the colon, and since transit time through the colon may be slow, 24 to 48 hours may be required to produce the desired bowel movement. Lactulose given orally to man and experimental animals resulted in only small amounts reaching the blood. Urinary excretion has been determined to be 3% or less and is essentially complete within 24 hours.