Characterization of the Placenta in the Newborn with Congenital Heart Disease: Distinctions Based on Type of Cardiac Malformation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fetal Blood Flow and Genetic Mutations in Conotruncal Congenital Heart Disease
Journal of Cardiovascular Development and Disease Review Fetal Blood Flow and Genetic Mutations in Conotruncal Congenital Heart Disease Laura A. Dyer 1 and Sandra Rugonyi 2,* 1 Department of Biology, University of Portland, Portland, OR 97203, USA; [email protected] 2 Department of Biomedical Engineering, Oregon Health & Science University, Portland, OR 97239, USA * Correspondence: [email protected] Abstract: In congenital heart disease, the presence of structural defects affects blood flow in the heart and circulation. However, because the fetal circulation bypasses the lungs, fetuses with cyanotic heart defects can survive in utero but need prompt intervention to survive after birth. Tetralogy of Fallot and persistent truncus arteriosus are two of the most significant conotruncal heart defects. In both defects, blood access to the lungs is restricted or non-existent, and babies with these critical conditions need intervention right after birth. While there are known genetic mutations that lead to these critical heart defects, early perturbations in blood flow can independently lead to critical heart defects. In this paper, we start by comparing the fetal circulation with the neonatal and adult circulation, and reviewing how altered fetal blood flow can be used as a diagnostic tool to plan interventions. We then look at known factors that lead to tetralogy of Fallot and persistent truncus arteriosus: namely early perturbations in blood flow and mutations within VEGF-related pathways. The interplay between physical and genetic factors means that any one alteration can cause significant disruptions during development and underscore our need to better understand the effects of both blood flow and flow-responsive genes. -

Fetal Circulation
The Fetal Circulation Dr. S. Mathieu, Specialist Registrar in Anaesthesia Dr. D. J. Dalgleish, Consultant Anaesthetist Royal Bournemouth and Christchurch Hospitals Trust, UK Questions 1. In the fetal circulation: a) There are two umbilical arteries and one umbilical vein? b) Over 90% of blood passes the liver via the ductus venosus c) The foramen ovale divides the left and right ventricle d) The umbilical artery carries oxygenated blood from the placenta to the fetus e) The foramen ovale allows oxygenated blood to bypass the pulmonary circulation 2. In the fetal circulation: a) The oxygen dissociation curve of fetal haemoglobin is shifted to the left compared with adult haemoglobin ensuring oxygen delivery to the fetus despite low oxygen partial pressures b) It is the presence of the ductus arteriosus and large pulmonary vascular resistance which ensures most of the right ventricular output passes into the aorta c) The patency of the ductus arteriosus is maintained by high oxygen tensions d) The patency of the ductus arteriosus is maintained by the vasodilating effects of prostaglandin G2 e) 2,3-DPG levels are higher in fetal haemoglobin compared with adult haemaglobin 3. Changes at birth include: a) a fall in pulmonary vascular resistance b) a rise in systemic vascular resistance with clamping of the cord c) an increase in hypoxic pulmonary vasoconstriction d) a rise in left atrial pressure e) closure of the ductus arteriosus within 24 hours 4. The following congenital heart lesions are cyanotic: a) Ventricular septal defect b) Atrial septal defect c) Patent ductus arteriosus d) Tetralogy of Fallot e) Transposition of the great arteries MCQ answers at end Key points • The fetal circulation supplies the fetal tissues with oxygen and nutrients from the placenta. -

Cardiovascular System Note: the Cardiovascular System Develops Early (Week 3), Enabling the Embryo to Grow Beyond the Short
Lymphatics: Lymph vessel formation is similar to blood angiogenesis. Lymphatics begin as lymph sacs in three regions: jugular (near brachiocephalic veins); cranial abdominal (future cysterna chyla); and iliac region. Lym- phatic vessels (ducts) form as outgrowths of the sacs. mesenchyme Lymph nodes are produced by localized mesoder- sinusoid lymph duct lumen mal invaginations that partition the vessel lumen into sinu- soids. The mesoderm develops a reticular framework within which mesodermal lymphocytes accumulate. The spleen and hemal nodes (in ruminants) invagination develop similar to the way lymph nodes develop. Lymph Node Formation Prior to birth, fetal circulation is designed for an in utero aqueous environment where the pla- centa oxygenates fetal blood. Suddenly, at birth... Three In-Utero Adjustments ductus Stretching and constriction of arteriosus umbilical arteries shifts fetal blood flow aortic arch from the placenta to the fetus. Reduced pulmonary trunk L atrium venous return through the (left) umbili- foramen ovale R cal vein and ductus venosus allows the atrium latter to gradually close (over a period caudal vena cava of days). Bradykinin released by expand- ductus venosus ing lungs and increased oxygen concen- tration in blood triggers constriction of aorta the ductus arteriosus which, over two liver months, is gradually converted to a fibrous structure, the ligamentum arte- umbilical v. riosum. portal v. The increased blood flow to the lungs and then to the left atrium equalizes pres- sure in the two atria, resulting in closure umbilical aa. of the foramen ovale that eventually grows permanent. 29 The cardiogenic area, the place where the embryonic heart originates, is located . -
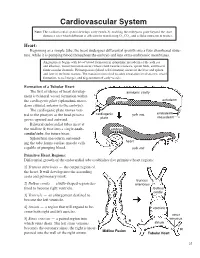
Cardiovascular System Note: the Cardiovascular System Develops Early (Week-3), Enabling the Embryo to Grow Beyond the Short
Cardiovascular System Note: The cardiovascular system develops early (week-3), enabling the embryo to grow beyond the short distances over which diffusion is efficient for transferring 2O , CO2, and cellular nutrients & wastes. Heart: Beginning as a simple tube, the heart undergoes differential growth into a four chambered struc- ture, while it is pumping blood throughout the embryo and into extra-embryonic membranes. Angiogenesis begins with blood island formation in splanchnic mesoderm of the yolk sac and allantois. Vessel formation occurs when island vesicles coalesce, sprout buds, and fuse to form vascular channels. Hematopoiesis (blood cell formation) occurs in the liver and spleen and later in the bone marrow. The transition from fetal to adult circulation involves new vessel formation, vessel merger, and degeneration of early vessels. Formation of a Tubular Heart: The first evidence of heart develop- amnionic cavity ment is bilateral vessel formation within ectoderm the cardiogenic plate (splanchnic meso- embryo derm situated anterior to the embryo). The cardiogenic plate moves ven- tral to the pharynx as the head process cardiogenic yolk sac endoderm mesoderm grows upward and outward. plate Bilateral endocardial tubes meet at the midline & fuse into a single endo- embryo cardial tube, the future heart. Splanchnic mesoderm surround- ing the tube forms cardiac muscle cells heart capable of pumping blood. yolk sac Primitive Heart Regions: Differential growth of the endocardial tube establishes five primitive heart regions: 1] Truncus arteriosus — the output region of the heart. It will develop into the ascending aorta and pulmonary trunk. truncus 2] Bulbus cordis — a bulb-shaped region des- arteriosus tined to become right ventricle. -

Truncus Arteriosus What the Nurse Caring for the Patient with Congenital Heart Disease Needs to Know
Truncus Arteriosus What the Nurse Caring for the Patient with Congenital Heart Disease Needs to Know Mary Rummell, MN, RN, CPNP, CNS, FAHA Clinical Nurse Specialist, Pediatric Cardiology, Cardiac Services, Oregon Health & Science University (Retired) Embryology Rare congenital heart disease Before the 4th week of embryonic life o Endocardial tube expands, elongates and develops areas of dilation . Includes bulbus cordis . Ventricular outflow tracks . Truncus arteriosus o Results in cardiac looping – completed by day 28 of gestation . Stretching creates torsion in truncus arteriosus . Contributes to formation of spiral septum Septation of the truncus arteriosus – day 26 to 42 o Mesenchymal cells . Include neural crest cells . Actively proliferate . Form ridges in bulbus cordis and truncus arteriosus . Ridges create 180 degree spiraling . Create aorticopulmonary septum . Contribute to closure of conal ventricular septum o Spiraling enhanced by forward blood flow o Failure of septation results in: . Truncus arteriosus (TA) . Large ventricular septal defect (VSD) Development of semilunar valves o At base of the truncus arteriosus . Result from: Swelling of endocardial tissue Endocardial cushions o Failure of septation . Results in one valve . Valve abnormal Usually have more than 3 cusps Cusps usually thickened and deformed May be regurgitant Sometimes stenotic Neural crest cells o Arise from genetic material o Influence the development of thymus and parathyroid glands from the pharyngeal pouches 1 o Result in increased prevalence -
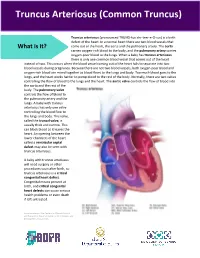
Truncus Arteriosus (Common Truncus) Truncus Arteriosus
Truncus Arteriosus (Common Truncus) Truncus Arteriosus Truncus arteriosus (pronounced TRUNG-kus ahr-teer-e-O-sus) is a birth defect of the heart. In a normal heart there are two blood vessels that What is it? come out of the heart, the aorta and the pulmonary artery. The aorta carries oxygen-rich blood to the body, and the pulmonary artery carries oxygen-poor blood to the lungs. When a baby has truncus arteriosus, there is only one common blood vessel that comes out of the heart instead of two. This occurs when the blood vessel coming out of the heart fails to separate into two blood vessels during pregnancy. Because there are not two blood vessels, both oxygen-poor blood and oxygen-rich blood are mixed together as blood flows to the lungs and body. Too much blood goes to the lungs, and the heart works harder to pump blood to the rest of the body. Normally, there are two valves controlling the flow of blood to the lungs and the heart. The aortic valve controls the flow of blood into the aorta and the rest of the body. The pulmonary valve controls the flow of blood to the pulmonary artery and the lungs. A baby with truncus arteriosus has only one valve controlling the blood flow to the lungs and body. This valve, called the truncal valve, is usually thick and narrow. This can block blood as it leaves the heart. An opening between the lower chambers of the heart called a ventricular septal defect may also be seen with truncus arteriosus. -

Aortic Arches
Human Embryology: Heart Development II Kimara L. Targoff, M.D. Division of Pediatric Cardiology, Columbia University Medical Center Developmental Genetics Program, Skirball Institute, NYU School of Medicine Human Vascular Development • Overview • Aortic Arch Development • Arterial Vascular Development • Venous System Development • Lymphatic Development • Transition from Fetal to Post-Natal Circulation Development of the Arterial and Venous Systems Cranial Ends of the Dorsal Aortae Form a Dorsoventral Loop: The First Aortic Arch Aortic Arches Arise in a Craniocaudal Sequence Surrounding the Pharynx Aortic Arches Give Rise to Important Head, Neck, and Upper Thorax Vessels Aortic Arch Development in the Chick Embryo Fgf8 is Required for Pharyngeal Arch Development in Mouse Abu-Issa, R. et al., Development 2002. Cardiovascular and Thymic Defects in Tbx1 Hypomorphic Mutant Neonates Hu, T. et al., Development 2004. Aortic Arch Development Dorsal aorta 1 2 3 Ventral aorta 4 5 6 7 iseg Harsh Thaker Aortic Arch Development Dorsal aorta 1 2 3 Ventral aorta 4 5 6 7 iseg Harsh Thaker Aortic Arch and Derivatives 3 3 4 4 7 iseg 6 7 iseg 6 Aortic sac Truncus arteriosus Harsh Thaker Aortic Arch and Derivatives 3 3 4 4 7 iseg 6 7 iseg Harsh Thaker Aortic Arch and Derivatives 3 3 4 7 iseg 4 7 iseg 6 Harsh Thaker Aortic Arch and Derivatives RCC LCC RSC LSC BCA DA Harsh Thaker Recurrent Laryngeal Nerves RCC LCC RSC LSC BCA DA Harsh Thaker Defects in Normal Regression of the Arterial System Lead to Vascular Anomalies • Double Aortic Arch – Failure of the -

Rare Variant Oftruncus Arteriosus with Intact Ventricular Septum and Hypoplastic Right Ventricle
214 Br Heart J 1992;68:214-5 CASE REPORTS Br Heart J: first published as 10.1136/hrt.68.8.214 on 1 August 1992. Downloaded from Rare variant of truncus arteriosus with intact ventricular septum and hypoplastic right ventricle Benjamin Zeevi, Leslie Dembo, Michael Berant Abstract showed an active precordium with a loud A three week old girl was admitted to systolic ejection click at the apex. The second hospital with severe congestive heart heart sound was loud and single. A grade 3/6 failure and cyanosis. Cross sectional and ejection systolic murmur was best heard at the Doppler echocardiography and cardiac apex and the left sternal border. A 2/6 mid- catheterisation showed a unique variant diastolic murmur was best heard at the apex. of truncus arteriosus with an intact ven- A chest x ray showed severe cardiomegaly tricular septum. The trunk rose only with increased pulmonary vascular markings. from the left ventricle and was asso- An electrocardiogram showed right atrial ciated with a hypoplastic right ventricle enlargement, left ventricular hypertrophy, with sinusoids to the right coronary and diffuse ST-T segment depression. artery. Cross sectional and Doppler echocardiogra- phy showed situs solitus and normal systemic (Br Heart J 1992;68:214-5) and pulmonary venous connections. The right atrium was very large with aneurysmal bulg- Truncus arteriosus is defined as a single ing of the interatrial septum into the left arterial trunk leaving the base of the heart via atrium. The tricuspid valve was hypoplastic a single arterial valve to supply the systemic, but patent and opened into a hypoplastic pulmonary, and coronary circulations.' A muscular right ventricle with sinusoids (fig truncus arteriosus with an intact ventricular 1A). -

Complex Congenital Heart Disease with Sacral Agenesis – a Case Report
1 International Journal of Anatomical Sciences 2011,2(1):1-6 Case Report Complex Congenital Heart disease with Sacral Agenesis – a Case Report Srimathi, T. Department of Anatomy, Sri Ramachandra University, Porur, Chennai 600 116, Tamil Nadu, India. Key Words: congenital heart disease, sacral agenesis Abstract: Complex congenital heart anomalies are less common. A thirty years old third gravida treated for secondary infertility, gestational diabetes mellitus and hypertension was admitted in Sri Ramachandra Medical College Hospital near term with a breech presentation of the fetus. Labour was induced due to Gestational diabetes and fetal bradycardia. She delivered by vaginal delivery / assisted breech delivery with episiotomy. The child had neonatal respiratory distress, did not cry at birth and was not breast feeding well. The child was treated in the neonatal Intensive care unit for the same. The foetal echocardiogram revealed small left ventricle, dilated right ventricle, single outflow from Right ventricle and probable Truncus arteriosus. Infantogram revealed cardiomegaly and Sacral Agenesis. The case is unique for its rarity and its embryological significance. Complex congenital heart diseases She was the third gravida with only Live are less common. A congenital heart defect born child in this conception. She had (CHD) is a defect in the structure of the Gestational diabetes mellitus and was on heart and great vessels that are present at insulin. She also had Pregnancy induced birth either obstruct blood flow in the heart hypertension and was on treatment. Patient or vessels near it or cause blood to flow was taking antenatal care elsewhere before through the heart in an abnormal pattern. -

Persistent Truncus Arteriosus
43993 Ganesh Elumalai and Abigail Adaeze Chinonyerem Onii Egbunine / Elixir Embryology 101 (2016) 43993-44000 Available online at www.elixirpublishers.com (Elixir International Journal) Embryology Elixir Embryology 101 (2016) 43993-44000 “PERSISTENT TRUNCUS ARTERIOSUS ” - EMBRYOLOGICAL BASIS AND ITS CLINICAL IMPORTANCE Ganesh Elumalai and Abigail Adaeze Chinonyerem Onii Egbunine Department of Embryology, College of Medicine, Texila American University, South America ARTICLE INFO ABSTRACT Article history: The Truncus Arteriosus (TA) is a vast developmental structure which is present during Received: 2 November 2016; fetal life and normally divides off to form two separate arteries, the Aorta and the Received in revised form: Pulmonary artery. In Persistent Truncus Arteriosus (PTA), the Truncus Arteriosus 4 December 2016; doesn‟t divide, giving rise to a single, large trunk, with a correlation between the Aorta Accepted: 16 December 2016; and Pulmonary artery, thereby allowing the mixture of oxygenated and deoxygenated blood in the Fetus. This article aims to discuss in the detail, the origin of this congenital Keywords heart anomaly and how it affects the population with focus on related clinical studies and Persistent Truncus Arteriosus possible remedies. (PTA), © 2016 Elixir All rights reserved. Truncus Arteriosus (TA), Interventricular Septal Defect (VSD), Aortico-pulmonary Septum, Aorta, Pulmonary Artery, Di George Syndrome (DGS), Truncal Valve. Introduction PTA is known as one of the rarer congenital cardiac As the fetal arterial system develops, it usually undergoes anomalies [5] frequently associated with interventricular complex stages which transform it into the adult arterial septal defect (VSDs) [10] in which affected infants usually pattern. One of these stages is the division of the Truncus present with mild cyanosis as well as signs of heart failure Arteriosus [1]. -

Cardiovascular System - Accessscience from Mcgraw-Hill Education
Cardiovascular system - AccessScience from McGraw-Hill Education http://accessscience.com/content/109900 (http://accessscience.com/) Article by: Weichert, Charles K. College of Arts and Sciences, University of Cincinnati, Cincinnati, Ohio. Copenhaver, W. M. College of Physicians and Surgeons, Columbia University, New York; Department of Biological Structures, School of Medicine, University of Miami, Miami, Florida. Ebert, James D. Department of Embryology, Carnegie Institution, Washington, DC. Patten, Bradley M. Department of Anatomy, University of Michigan, Ann Arbor, Michigan. Jones, David R. Department of Zoology, University of British Columbia, Vancouver, Canada. Publication year: 2014 DOI: http://dx.doi.org/10.1036/1097-8542.109900 (http://dx.doi.org/10.1036/1097-8542.109900) Content Comparative Anatomy Embryogenesis of blood vessels Balancing ventricular output Heart Angiogenesis Human Postnatal Circulation Arterial system Circulatory system morphogenesis Pulmonary circuit and ductus Venous system Primitive venous system Physiological aspects of transition Comparative Embryology Functional Development of Heart Comparative Physiology Heart Contractions of the heart General physiology of circulation Tubular heart formation Heart-forming areas Microcirculation Cardiac loop and regional development Contractile proteins Heart Formation of definitive heart Synthesis of contractile proteins Arteries Partitioning of mammalian heart Action of inhibitors Venous system Division of atrium and ventricles Human Fetal Circulation at Term Bibliography -

Outcome of Fetuses with Heart Disease Diagnosed in Utero
Archives of Disease in Childhood 1997;77:F41–F46 F41 Arch Dis Child Fetal Neonatal Ed: first published as 10.1136/fn.77.1.F41 on 1 July 1997. Downloaded from Outcome of fetuses with heart disease diagnosed in utero Marianne Eronen Abstract Notable exceptions are the atrial septal defect, Objective—To review the outcomes of 193 because of the extreme thinness and the fetuses with cardiac abnormalities de- presence of the patent foramen ovale of the tected by echocardiography. atrial septum in the fetus, and the arterial duct, Methods—A total of 422 fetuses between 16 which is a normal finding. Some defects can and 41 gestational weeks, referred to pae- also evolve or progress during fetal life, making diatric cardiologists for detailed echocar- a definitive diagnosis early in gestation diography, were included in this study. diYcult.7 Prenatal diagnosis not only allows for Results—Structural heart defects were planned management of the heart disease pre- found in 55 (28%), isolated arrhythmia in natally and postnatally, but also allows the 105 (54%), and other non-structural ab- families to consider the option of termination normalities (dilated cardiomyopathy, of pregnancy for severe defects. hypertrophic cardiomyopathy, aneurysm Echocardiographic assessment of fetal ar- of the foramen ovale, isolated pericardial rhythmias is usually accomplished using eVusion or echogenic foci) in 33 (17%) of M-mode and Doppler techniques. Echocardio- 193 fetuses. Total mortality was 26%. The graphy not only establishes the kind of arrhyth- prognosis was poor in fetuses with struc- mia present but can also identify associated tural heart defects; 37 of 55 cases (67%) structural and functional heart disease as well.