(19) United States (12) Patent Application Publication (10) Pub
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
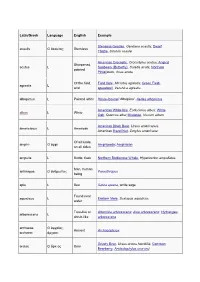
Dwarf Thistle, Cirsi
Latin/Greek Language English Example Stemless Gentian, Gentiana acaulis; Dwarf acaulis G ἄκαυλος Stemless Thistle, Cirsium acaule American Crocodile, Crocodylus acutus; Angled Sharpened, acutus L Sunbeam (Butterfly), Curetis acuta; Northern pointed Pintailduck, Anas acuta Of the field, Field Vole, Microtus agrestis; Green Field- agrestis L wild speedwell, Veronica agrestis albopictus L Painted white Hosta fortunei 'Albopicta', Aedes albopictus American White Ibis, Eudocimus albus; White albus L White Oak, Quercus alba; Mistletoe, Viscum album American Black Bear, Ursus americanus; americanus L American American Hazel Nut, Corylus americana Of all kinds, amphi- G ἀμφί Amphipoda; Amphibian on all sides ampulla L Bottle, flask Northern Bottlenose Whale, Hyperoodon ampullatus Man, human anthropos G ἄνθρωπος Paranthropus being apis L Bee Salvia apiana, white sage Found near aquaticus L Eastern Mole, Scalopus aquaticus water Tree-like or Artemisia arborescens; Aloe arborescens; Hydrangea arborescens L shrub-like arborescens archaeos, G ἀρχαῖος, Ancient Archaeopteryx archaeo- ἀρχαιο- Grizzly Bear, Ursus arctos horribilis; Common arctos G ἄρκτος Bear Bearberry, Arctostaphylos uva-ursi argentatus L Silvery Herring Gull, Larus argentatus arthron G ἄρθρον Joint Arthropoda arvensis L In the field Skylark, Alauda arvensis astron, astro-, G ἄστρον, Star Starfish (class), Asteroidea astero- ἀστρο-, ἀστερο- Acer palmatum 'Atropurpureum'; Berberis atropurpureum L Deep purple thunbergii f. atropurpurea Daphne odora 'Aureomarginata'; Taxus aureomarginata -

North American Rock Garden Society |
Bulletin of the American Rock Garden Society Volume 50 Number 4 Fall 1992 Cover: Gentiana paradoxa by Rob Proctor of Denver, Colorado Bulletin of the American Rock Garden Society Volume 50 Number 4 Fall 1992 Features Sorting out the Gentians, by Geoffrey Charlesworth 243 Fritillaries of Central Asia, by Josef Slegl 253 Trillium Rescue, by Don L. Jacobs 261 The Story of Fritillaria 'Martha Roderick', by W.H. de Goede 264 New Home for Rock Plants, by Elisabeth Sheldon 265 Eriogonums: Secret of the Dry Garden, by Irma Gourley 271 Preserving Rock Garden Specimens, by Karen Matthews 275 Spontaneity on the Rocks, by Panayoti Kelaidis 285 The Arctic Harebell, by J.S. DeSanto 291 Hunting for Red Helleborus niger, by Will McLewin 295 Departments Plant Portrait: Gentiana paradoxa 276 Awards 299 Books 305 Gentiana algida 242 Bulletin of the American Rock Garden Society Vol. 50(4) Sorting out the Gentians by Geoffrey Charlesworth 1 here are some genera in which tors. It is one of the hallmarks of a many of the species are considered good grower if a large patch can be good alpine plants. Androsace is such produced and maintained year after a genus, and we tend to dismiss the year, but the despair of most of us, who species that are not up to the highest have only occasionally seen a few small standard as not worth growing—for plants in our own gardens and then not instance, A. loctiflora or A. albana. It always with the astonishing color we is a mistake to make such odious associate with the species. -

Phylogeny and Phylogenetic Taxonomy of Dipsacales, with Special Reference to Sinadoxa and Tetradoxa (Adoxaceae)
PHYLOGENY AND PHYLOGENETIC TAXONOMY OF DIPSACALES, WITH SPECIAL REFERENCE TO SINADOXA AND TETRADOXA (ADOXACEAE) MICHAEL J. DONOGHUE,1 TORSTEN ERIKSSON,2 PATRICK A. REEVES,3 AND RICHARD G. OLMSTEAD 3 Abstract. To further clarify phylogenetic relationships within Dipsacales,we analyzed new and previously pub- lished rbcL sequences, alone and in combination with morphological data. We also examined relationships within Adoxaceae using rbcL and nuclear ribosomal internal transcribed spacer (ITS) sequences. We conclude from these analyses that Dipsacales comprise two major lineages:Adoxaceae and Caprifoliaceae (sensu Judd et al.,1994), which both contain elements of traditional Caprifoliaceae.Within Adoxaceae, the following relation- ships are strongly supported: (Viburnum (Sambucus (Sinadoxa (Tetradoxa, Adoxa)))). Combined analyses of C ap ri foliaceae yield the fo l l ow i n g : ( C ap ri folieae (Diervilleae (Linnaeeae (Morinaceae (Dipsacaceae (Triplostegia,Valerianaceae)))))). On the basis of these results we provide phylogenetic definitions for the names of several major clades. Within Adoxaceae, Adoxina refers to the clade including Sinadoxa, Tetradoxa, and Adoxa.This lineage is marked by herbaceous habit, reduction in the number of perianth parts,nectaries of mul- ticellular hairs on the perianth,and bifid stamens. The clade including Morinaceae,Valerianaceae, Triplostegia, and Dipsacaceae is here named Valerina. Probable synapomorphies include herbaceousness,presence of an epi- calyx (lost or modified in Valerianaceae), reduced endosperm,and distinctive chemistry, including production of monoterpenoids. The clade containing Valerina plus Linnaeeae we name Linnina. This lineage is distinguished by reduction to four (or fewer) stamens, by abortion of two of the three carpels,and possibly by supernumerary inflorescences bracts. Keywords: Adoxaceae, Caprifoliaceae, Dipsacales, ITS, morphological characters, phylogeny, phylogenetic taxonomy, phylogenetic nomenclature, rbcL, Sinadoxa, Tetradoxa. -

Some Medicinal Plants from Wild Flora of Romania and the Ecology
Research Journal of Agricultural Science, 44 (2), 2012 SOME MEDICINAL PLANTS FROM WILD FLORA OF ROMANIA AND THE ECOLOGY Helena Maria SABO Faculty of Psychology and Science of Education, UBB, Sindicatelor Street. No.7, Cluj-Napoca, Romania E-mail: [email protected] Abstract: The importance of ecological factors for characteristic of central and Western Europe, medicinal species and their influence on active specific continental to the Eastern Europe, the principles synthesis and the specific uptake of presence of the Carpathian Mountains has an mineral elements from soil are presented. The impact on natural vegetation, and vegetation in the biological and ecological characters, the medicinal south has small Mediterranean influence. The importance, and the protection measurements for therapeutic use of medicinal plants is due to active some species are given. Ecological knowledge of principles they contain. For the plant body these medicinal plants has a double significance: on the substances meet have a metabolic role, such as one hand provides information on resorts where vitamins, enzymes, or the role of defense against medicinal plant species can be found to harvest and biological agents (insects, fungi, even vertebrates) use of them, on the other hand provides to chemical and physical stress (UV radiation), and information on conditions to be met by a possible in some cases still not precisely known functions of location of their culture. Lately several medicinal these substances for plants. As a result of research species were introduced into culture in order to on medicinal plants has been established that the ensure the raw materials of vegetable drug following factors influence ecology them: abiotic - industry. -

New Distributional Record of Gentiana Tetrasepala Biswas (Gentianales
JoTT NOTE 3(9): 2100–2103 New distributional record of Gentiana meadows of the Valley of Flowers tetrasepala Biswas (Gentianales: National Park (VoFNP) (Image Gentianaceae) from the Valley of 1), we report here the recollection Flowers National Park, Garhwal of Gentiana tetrasepala Biswas Himalaya along with the causes of recent threats and the high need of conservation. C.S. Rana 1, V. Rana 2 & M.P.S. Bisht 3 Gentiana tetrasepala was described by Biswas in 1938 on the basis of specimens collected by J.F. 1 State Medicinal Plants Board Uttarakhand, Dehradun, Uttarakhand 248006, India, Duthie (No. 3166 CAL) from Ralam Valley (Kumaon 2,3 Department of Geology, HNB Garhwal University, Srinagar Garhwal) on 26 August 1884. Since then the species (Garhwal), Uttarakhand 246174, India Email: 1 [email protected] (corresponding author), was never recorded leading to the general assumption 2 [email protected], 3 [email protected] that the species had either become extinct or is not a distinct and taxonomically valid species (Garg 1987). Chowdhery & Murti (2000) mentioned this Endemic plants are more prone to extinction for species among the red taxa as per IUCN’s criteria of various reasons as they are habitat specific. Because red taxa (IUCN 1994). It was placed under the ‘IK’ of unstable habitats, in a small area with a limited (insufficiently known) category as per the Indian population they are extra stressed. Therefore, such Red Data Book (Nayar & Shastry 1987). Rawat endemics must be prioritized for conservation efforts (2009) suggested that it be placed under the category (Rawat 2009). Considering this, we have been trying ‘I’ (indeterminate). -

Recovery Plan
Approved Recovery Plan Recovery Plan for the “lost” threatened flora of south-eastern NSW Baeuerlen’s Gentian (Gentiana baeuerlenii), Elusive Bush-pea (Pultenaea parrisiae subsp. elusa), Elusive Cress (Irenepharsus magicus), Formbe Peppercress (Lepidium pseudopapillosum), Hidden Violet (Viola cleistogamoides), Mueller’s Eyebright (Euphrasia collina subsp. muelleri), Rosella Spider Orchid (Caladenia rosella), and Swamp Groundsel (Senecio squarrosus) NSW NATIONAL PARKS AND WILDLIFE July 2001 SERVICE © NSW National Parks and Wildlife Service, 2001. This work is copyright. However, material printed in this plan may be copied for personal use or published for educational purposes, provided that any extracts are fully acknowledged. Apart from any use as permitted under the Copyright Act 1968, no part may be reproduced without prior written permission from NPWS. NSW National Parks and Wildlife Service 43 Bridge Street (PO Box 1967) Hurstville NSW 2220 Tel: 02 95856444 www.npws.nsw.gov.au For further information contact Threatened Species Unit, Southern Directorate NSW National Parks and Wildlife Service PO Box 2115 Queanbeyan NSW 2620 Tel (02) 629 89700 Cover photo: Euphrasia collina subsp. paludosa, a species similar in appearance to Mueller’s Eyebright Photographer: Colin Totterdell This plan should be cited as: NSW National Parks and Wildlife Service (2001) Approved Recovery Plan for the “lost” threatened flora of south-eastern NSW. NSW NPWS, Hurstville, NSW. ISBN: 0 7313 6205 5 Approved Recovery Plan Lost threatened flora of SE NSW Recovery Plan for the “lost” threatened flora of south-eastern NSW: Baeuerlen’s Gentian (Gentiana baeuerlenii), Elusive Bush-pea (Pultenaea parrisiae subsp. elusa), Elusive Cress (Irenepharsus magicus), Formbe Peppercress (Lepidium pseudopapillosum), Hidden Violet (Viola cleistogamoides), Mueller’s Eyebright (Euphrasia collina subsp. -

Materia Medica of Tibetan Medicine: Identification, Quality Check And
Asian Medicine 5 (2009) 407–432 brill.nl/asme Materia Medica of Tibetan Medicine: Identification, Quality Check and Protection Measures Dr Dawa Translation by Tsering D. Gonkatsang Abstract This paper discusses the relationship between Tibetan medical theory and practice with respect to the classification of materia medica and the discernment of quality and potency. Based on more than thirty years of experience as a Tibetan medical practitioner, the author describes a number of specific materia medica in detail, with an emphasis on how to determine fake from authentic ingredients. The author also offers recommendations and guidance on proper cultiva- tion techniques and conservation methods, in line with Tibetan textual sources on the subject, in combination with empirical knowledge. Keywords Tibetan medicine, materia medica, quality, cultivation, threats to species diversity Editors’ Introduction As one of the world’s leading experts on Tibetan materia medica, the author of the seminal Tibetan and English publication A Clear Mirror of Medicinal Plants (Rome: Tibet Domani, 2002) and a former director of the Men-Tsee-Khang in Dharamsala, India, Dr Dawa is in a unique position to comment not only on issues of species identification, potency, and quality, but also on much broader social and ethical concerns with respect to the current and future status of Tibetan medicinal plants, and the practice of gso ba rig pa. At a basic level, Dr Dawa is addressing here the protection of plants as well as issues of classification and discernment with respect to quality, potency, and efficacy of materia medica. More significantly, though, he is addressing the protection of gso ba rig pa as a whole. -

Gentiana L. Spp. Gentian Gentianaceae GENTI
Plants Gentiana L. spp. Gentian Gentianaceae GENTI G. sceptrum Griseb., King’s gentian-GESC G. calycosa Griseb., Explorer’s gentian-GESA Ecology Description: Native. About 300 species, 36 in Western United States; annual or perennial herb; simple stems; fleshy roots or slender rhizomes; opposite, occasionally whorled, often clasping leaves; inflorescence compact cyme or solitary flowers, bell or funnel shaped, four or five lobed corollas, blue, violet purple, greenish, yellow, red or white; capsule, two valved, many seeded. Genti- ana sceptrum, 25-100 cm, leaves 10 to 15, 3-6 cm, blue 3-4.5 cm flowers; G. calycosa, 5-30 cm. Range and distribution: Temperate to subarctic and alpine America and Eurasia. Gentiana sceptrum: from British Columbia to California, western slope of Cas- cade Range to coast; G. calycosa: also to Rocky Moun- Gentiana calycosa G. sceptrum tains. Widespread and common for some species; others locally abundant. Biology Associations: Sitka spruce, western hemlock, Pacific Flowering and fruiting: Gentiana sceptrum blooms silver fir zones. Western redcedar, alder, willow, black from July through September, G. calycosa from July cottonwood, and bog or moist meadow. Gentiana caly- through October. cosa: mountain heather, black huckleberry, broadleaf lupine, and showy sedge. Seed: Abundant seed producer; seeds small; disperse well. Seeds are sown in autumn to early spring on top Habitat: Meadows; G. calycosa, moist open sites in of well-drained, sandy soil, watered from beneath. mountains; other gentian species including G. sceptrum, Germination in full sunlight. lower foothills and near coast. Vegetative reproduction: May be rooted from stem Successional stage: Component of well-developed, cuttings; difficult to start. -

On the Flora of Australia
L'IBRARY'OF THE GRAY HERBARIUM HARVARD UNIVERSITY. BOUGHT. THE FLORA OF AUSTRALIA, ITS ORIGIN, AFFINITIES, AND DISTRIBUTION; BEING AN TO THE FLORA OF TASMANIA. BY JOSEPH DALTON HOOKER, M.D., F.R.S., L.S., & G.S.; LATE BOTANIST TO THE ANTARCTIC EXPEDITION. LONDON : LOVELL REEVE, HENRIETTA STREET, COVENT GARDEN. r^/f'ORElGN&ENGLISH' <^ . 1859. i^\BOOKSELLERS^.- PR 2G 1.912 Gray Herbarium Harvard University ON THE FLORA OF AUSTRALIA ITS ORIGIN, AFFINITIES, AND DISTRIBUTION. I I / ON THE FLORA OF AUSTRALIA, ITS ORIGIN, AFFINITIES, AND DISTRIBUTION; BEIKG AN TO THE FLORA OF TASMANIA. BY JOSEPH DALTON HOOKER, M.D., F.R.S., L.S., & G.S.; LATE BOTANIST TO THE ANTARCTIC EXPEDITION. Reprinted from the JJotany of the Antarctic Expedition, Part III., Flora of Tasmania, Vol. I. LONDON : LOVELL REEVE, HENRIETTA STREET, COVENT GARDEN. 1859. PRINTED BY JOHN EDWARD TAYLOR, LITTLE QUEEN STREET, LINCOLN'S INN FIELDS. CONTENTS OF THE INTRODUCTORY ESSAY. § i. Preliminary Remarks. PAGE Sources of Information, published and unpublished, materials, collections, etc i Object of arranging them to discuss the Origin, Peculiarities, and Distribution of the Vegetation of Australia, and to regard them in relation to the views of Darwin and others, on the Creation of Species .... iii^ § 2. On the General Phenomena of Variation in the Vegetable Kingdom. All plants more or less variable ; rate, extent, and nature of variability ; differences of amount and degree in different natural groups of plants v Parallelism of features of variability in different groups of individuals (varieties, species, genera, etc.), and in wild and cultivated plants vii Variation a centrifugal force ; the tendency in the progeny of varieties being to depart further from their original types, not to revert to them viii Effects of cross-impregnation and hybridization ultimately favourable to permanence of specific character x Darwin's Theory of Natural Selection ; — its effects on variable organisms under varying conditions is to give a temporary stability to races, species, genera, etc xi § 3. -

Gentiana Wingecarribiensis
THREATENED SPECIES INFORMATION Gentiana wingecarribiensis Common name Wingecarribee Gentian Conservation status Distribution Gentiana wingecarribiensis L. G. Adams G. wingecarribiensis is endemic to New is listed as an Endangered Species on South Wales and is known from only two Schedule 1 of the New South Wales localities. The type locality is Wingecarribee Threatened Species Conservation Act, Swamp, near Robertson on the Central 1995. This species is also listed as an Tablelands of New South Wales (Harden Endangered Species on Schedule 1 of the 1992), where two-three discrete patches Commonwealth Endangered Species occur on the southern side of the swamp Protection Act, 1992. (Kodela et al. 1994; J. Briggs pers. comm.). A second location was discovered in 1994 General description at Hanging Rock Swamp, 40 km south-west The small shrub, G. wingecarribiensis, has of Wingecarribee Swamp. Four discrete greenish ribbed flowers which are sky blue patches have been found at this site (Matthes inside. Flowers are generally only open in et al. 1996). direct sun from October to December. The stem is a reddish colour and leaves are broad Recorded occurrences in and oval. Photographs of G. conservation reserves wingecarribiensis and its habitat are G. wingecarribiensis is not known to occur provided in Cohn (1993) and an illustration in any conservation reserves (Briggs & can be found in Harden (1992). Leigh 1996). Scientific description Habitat G. wingecarribiensis (Gentianaceae) is an At Wingecarribee Swamp, G. erect (4-9 cm tall), annual (possibly biennial) wingecarribiensis is located in an ecotone hairless herb. Its stem is simple or sparsely area within 10-15 m of the swamp margin branched and reddish tinged. -

Research Journal of Pharmaceutical, Biological and Chemical Sciences
ISSN: 0975-8585 Research Journal of Pharmaceutical, Biological and Chemical Sciences Obtaining and Research of Callus Mass of Gentiana lutea L Roots. RT Konechna1*, Yu T Konechnyi2, RO Petrina1, RH Shykula2, Wieczorek P3, Jasicka-Misiak I3, and V P Novikov1. 1Lviv Polytechnic National University, Department of Technology of Biologically Active Compounds, Pharmacy and Biotechnology, Ukraine. 2Danylo Halytsky Lviv National Medical University, Department of Microbiology, Immunology and Virology, Ukraine. 3University of Opole, Faculty of Chemistry, Department Analytical and Ecological Chemistry, Poland. ABSTRACT The results of introduction to the culture in vitro Gentiana lutea L. are provided. The influence of phytohormones on growth processes of plant cells is researched, the optimal conditions for cultivation of Gentiana lutea L. are defined and chosen. Biomass extract is obtained and is researched on the presence in it the biologically active substances and antimicrobial activity. Keywords: Gentiana lutea L., callusogenesis, phytohormones, callus, explant, extract, antimicrobial activity. *Corresponding author July–August 2015 RJPBCS 6(4) Page No. 1490 ISSN: 0975-8585 INTRODUCTION Currently, important research is that, which provide us with the new range of medicines based on medicinal plants. Search for the advanced plants among native flora is today an urgent task of modern pharmacy. Interesting objects of study include representatives of family Gentianaceae, namely Gentiana lutea L. Gentiana lutea L. – this is unique, very rare, one of the most popular medicinal herbs of Ukrainian Carpathians, which is extensively used by official and folk medicine. Perennial herb of 50-120 cm height may be met in Ukraine only in Carpathians, namely - Chornohora, Svydovets, Marmaroski Alpy, Polonyna Borzhava, Horhany [7,13]. -

Alpine Garden Club of BC 26 SPRING 2008
Alpine Garden Club of British Columbia Vol.51 No. 2 Bulletin SPRING 2008 Web Address: www.agc-bc.ca President: Linda Verbeek 1st V.P. Philip MacDougall 2nd V.P. Dave Sellars Secretary: Allison Carson Treasurer: Amanda Offers Past President: Doug Smith Membership: Ian Gillam Seed Exchange: Ian and Phyllis Plenderleith Program: Philip MacDougall Annual Show: Diana Hume, Karen Thirkell Pot Show: Dana Cromie Plant Sale: Mark Demers Librarian: Pam Frost Open Gardens: Ann Dies Refreshments: Dorothy Yarema Publicity: Joan Bunn Webmaster: Chris Klapwijk Bulletin Editor: Sue Evanetz Bulletin Publishing: Moya Drummond Copy Editor: Ian Gillam Committee Members Mark Demers, Ann Dies, Diana Hume, Stuart Scholefield Karen Thirkell, Chris Klapwijk Honorary Life Members Margaret Charlton, Grace Conboy, Francisca Darts, Pam Frost, Daphne Guernsey, Bodil Leamy, Jim MacPhail, Geoff Williams, Bob Woodward Meetings are held the second Wednesday of each month except July & August, in the Floral Hall, VanDusen Botanical Garden. Doors and Library open at 7:00pm and Meetings start at 7:30pm with the educational talk. Don’t forget to bring a prize for the raffle which goes a long way to paying for the hall rental. Cover: Recently I fell heir to a number of back issues of the Bulletin dating from 1992 collected by past Honorary Life Member Frank Dorsey. What a wealth of articles and drawings; not to mention a sort of condensed history of our Club. The cover drawings are all by members, sadly some of whom are no longer with us. However, it is our intention to have a gallery of our members’ drawings on our website as a permanent record and I am starting this month to share with you some of these treasures.