Protocol for Efficient Micropropagation of Spring Gentian and Sand Jurinea
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download Chapter In
Flora and vegetation Margaret E Bradshaw The flora of Upper Teesdale is probably more widely known than that of any other area in Britain, and yet perhaps only a few of the thousands who visit the Dale each year realise the extent to which the vegetation and flora contribute to the essence of its character. In the valley, the meadows in the small walled fields extend, in the lower part, far up the south-facing slope, and, until 1957 to almost 570m at Grass Hill, then the highest farm in England. On the north face, the ascent of the meadows is abruptly cut off from the higher, browner fells by the Whin Sill cliff, marked by a line of quarries. Below High Force, the floor of the valley has a general wooded appearance which is provided by the small copses and the many isolated trees growing along the walls and bordering the river. Above High Force is a broader, barer valley which merges with the expansive fells leading up to the characteristic skyline of Great Dun Fell, Little Dun Fell and Cross Fell. Pennine skyline above Calcareous grassland and wet bog, Spring gentian Red Sike Moss © Margaret E Bradshaw © Geoff Herbert Within this region of fairly typical North Pennine vegetation is a comparatively small area which contains many species of flowering plants, ferns, mosses, liverworts and lichens which can be justifiably described as rare. The best known is, of course, the spring gentian (Gentiana verna), but this is only one of a remarkable collection of plants of outstanding scientific value. -
Dwarf Thistle, Cirsi
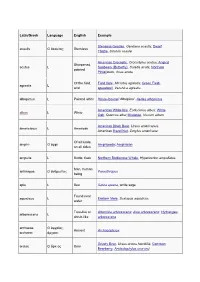
Latin/Greek Language English Example Stemless Gentian, Gentiana acaulis; Dwarf acaulis G ἄκαυλος Stemless Thistle, Cirsium acaule American Crocodile, Crocodylus acutus; Angled Sharpened, acutus L Sunbeam (Butterfly), Curetis acuta; Northern pointed Pintailduck, Anas acuta Of the field, Field Vole, Microtus agrestis; Green Field- agrestis L wild speedwell, Veronica agrestis albopictus L Painted white Hosta fortunei 'Albopicta', Aedes albopictus American White Ibis, Eudocimus albus; White albus L White Oak, Quercus alba; Mistletoe, Viscum album American Black Bear, Ursus americanus; americanus L American American Hazel Nut, Corylus americana Of all kinds, amphi- G ἀμφί Amphipoda; Amphibian on all sides ampulla L Bottle, flask Northern Bottlenose Whale, Hyperoodon ampullatus Man, human anthropos G ἄνθρωπος Paranthropus being apis L Bee Salvia apiana, white sage Found near aquaticus L Eastern Mole, Scalopus aquaticus water Tree-like or Artemisia arborescens; Aloe arborescens; Hydrangea arborescens L shrub-like arborescens archaeos, G ἀρχαῖος, Ancient Archaeopteryx archaeo- ἀρχαιο- Grizzly Bear, Ursus arctos horribilis; Common arctos G ἄρκτος Bear Bearberry, Arctostaphylos uva-ursi argentatus L Silvery Herring Gull, Larus argentatus arthron G ἄρθρον Joint Arthropoda arvensis L In the field Skylark, Alauda arvensis astron, astro-, G ἄστρον, Star Starfish (class), Asteroidea astero- ἀστρο-, ἀστερο- Acer palmatum 'Atropurpureum'; Berberis atropurpureum L Deep purple thunbergii f. atropurpurea Daphne odora 'Aureomarginata'; Taxus aureomarginata -

North American Rock Garden Society |
Bulletin of the American Rock Garden Society Volume 50 Number 4 Fall 1992 Cover: Gentiana paradoxa by Rob Proctor of Denver, Colorado Bulletin of the American Rock Garden Society Volume 50 Number 4 Fall 1992 Features Sorting out the Gentians, by Geoffrey Charlesworth 243 Fritillaries of Central Asia, by Josef Slegl 253 Trillium Rescue, by Don L. Jacobs 261 The Story of Fritillaria 'Martha Roderick', by W.H. de Goede 264 New Home for Rock Plants, by Elisabeth Sheldon 265 Eriogonums: Secret of the Dry Garden, by Irma Gourley 271 Preserving Rock Garden Specimens, by Karen Matthews 275 Spontaneity on the Rocks, by Panayoti Kelaidis 285 The Arctic Harebell, by J.S. DeSanto 291 Hunting for Red Helleborus niger, by Will McLewin 295 Departments Plant Portrait: Gentiana paradoxa 276 Awards 299 Books 305 Gentiana algida 242 Bulletin of the American Rock Garden Society Vol. 50(4) Sorting out the Gentians by Geoffrey Charlesworth 1 here are some genera in which tors. It is one of the hallmarks of a many of the species are considered good grower if a large patch can be good alpine plants. Androsace is such produced and maintained year after a genus, and we tend to dismiss the year, but the despair of most of us, who species that are not up to the highest have only occasionally seen a few small standard as not worth growing—for plants in our own gardens and then not instance, A. loctiflora or A. albana. It always with the astonishing color we is a mistake to make such odious associate with the species. -

Phylogeny and Phylogenetic Taxonomy of Dipsacales, with Special Reference to Sinadoxa and Tetradoxa (Adoxaceae)
PHYLOGENY AND PHYLOGENETIC TAXONOMY OF DIPSACALES, WITH SPECIAL REFERENCE TO SINADOXA AND TETRADOXA (ADOXACEAE) MICHAEL J. DONOGHUE,1 TORSTEN ERIKSSON,2 PATRICK A. REEVES,3 AND RICHARD G. OLMSTEAD 3 Abstract. To further clarify phylogenetic relationships within Dipsacales,we analyzed new and previously pub- lished rbcL sequences, alone and in combination with morphological data. We also examined relationships within Adoxaceae using rbcL and nuclear ribosomal internal transcribed spacer (ITS) sequences. We conclude from these analyses that Dipsacales comprise two major lineages:Adoxaceae and Caprifoliaceae (sensu Judd et al.,1994), which both contain elements of traditional Caprifoliaceae.Within Adoxaceae, the following relation- ships are strongly supported: (Viburnum (Sambucus (Sinadoxa (Tetradoxa, Adoxa)))). Combined analyses of C ap ri foliaceae yield the fo l l ow i n g : ( C ap ri folieae (Diervilleae (Linnaeeae (Morinaceae (Dipsacaceae (Triplostegia,Valerianaceae)))))). On the basis of these results we provide phylogenetic definitions for the names of several major clades. Within Adoxaceae, Adoxina refers to the clade including Sinadoxa, Tetradoxa, and Adoxa.This lineage is marked by herbaceous habit, reduction in the number of perianth parts,nectaries of mul- ticellular hairs on the perianth,and bifid stamens. The clade including Morinaceae,Valerianaceae, Triplostegia, and Dipsacaceae is here named Valerina. Probable synapomorphies include herbaceousness,presence of an epi- calyx (lost or modified in Valerianaceae), reduced endosperm,and distinctive chemistry, including production of monoterpenoids. The clade containing Valerina plus Linnaeeae we name Linnina. This lineage is distinguished by reduction to four (or fewer) stamens, by abortion of two of the three carpels,and possibly by supernumerary inflorescences bracts. Keywords: Adoxaceae, Caprifoliaceae, Dipsacales, ITS, morphological characters, phylogeny, phylogenetic taxonomy, phylogenetic nomenclature, rbcL, Sinadoxa, Tetradoxa. -

Some Medicinal Plants from Wild Flora of Romania and the Ecology
Research Journal of Agricultural Science, 44 (2), 2012 SOME MEDICINAL PLANTS FROM WILD FLORA OF ROMANIA AND THE ECOLOGY Helena Maria SABO Faculty of Psychology and Science of Education, UBB, Sindicatelor Street. No.7, Cluj-Napoca, Romania E-mail: [email protected] Abstract: The importance of ecological factors for characteristic of central and Western Europe, medicinal species and their influence on active specific continental to the Eastern Europe, the principles synthesis and the specific uptake of presence of the Carpathian Mountains has an mineral elements from soil are presented. The impact on natural vegetation, and vegetation in the biological and ecological characters, the medicinal south has small Mediterranean influence. The importance, and the protection measurements for therapeutic use of medicinal plants is due to active some species are given. Ecological knowledge of principles they contain. For the plant body these medicinal plants has a double significance: on the substances meet have a metabolic role, such as one hand provides information on resorts where vitamins, enzymes, or the role of defense against medicinal plant species can be found to harvest and biological agents (insects, fungi, even vertebrates) use of them, on the other hand provides to chemical and physical stress (UV radiation), and information on conditions to be met by a possible in some cases still not precisely known functions of location of their culture. Lately several medicinal these substances for plants. As a result of research species were introduced into culture in order to on medicinal plants has been established that the ensure the raw materials of vegetable drug following factors influence ecology them: abiotic - industry. -

(19) United States (12) Patent Application Publication (10) Pub
US 20110206787A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2011/0206787 A1 West et al. (43) Pub. Date: Aug. 25, 2011 (54) MORINDA CITRIFOLIA AND IRIDOID (60) Provisional application No. 61/307,262, ?led on Feb. BASED FORMULATIONS 23, 2010, provisional application No. 60/536,663, ?led on Jan. 15, 2004, provisional application No. (76) Inventors: Brett Justin West, Cedar Hills, UT 60/552,144, ?led on Mar, 10, 2004, provisional appli (US); Claude Jarakae Jensen, cation No. 60/335,343, ?led on Nov. 2, 2001, provi Cedar Hills, UT (Us); Afa Kehaati sional application No. 60/251,416, ?led on Dec. 5, Palu, American Fork, UT (US); 2000' ShiXin Deng, Lehi, UT (U S); Jlelfsfery A‘ Wasden’ Springv 111e, UT Publication Classi?cation ( ) (51) Int. Cl. (21) Appl. No.: 13/032,540 A61K 36/18 (200601) A61K 36/87 (2006.01) (22) Filed: Feb. 22, 2011 A61K 36/45 (2006.01) A61K 36/63 (2006.01) Related US. Application Data A61K 36/73 (2006.01) (60) Continuation-in-part of application No. 11/034,505, (200601) ?led on Jan. 13, 2005, Contmuatlon-m-part. of appli-. A611, 3/10 (2006(2006.01) 01) cationNo. 09/836,881, ?led onApr. 17, 2001 , noW Pat. ' No. 6,737,089, Continuation-in-part of application (52) US. Cl. ....... .. 424/732; 424/725; 424/774; 424/777; No. 11/253,130, ?led on Oct. 18, 2005, noW Pat. No. 424/773; 424/766; 424/765 7,244,463, Continuation-in-part of application No. 10/396,868, ?led on Mar. -

New Distributional Record of Gentiana Tetrasepala Biswas (Gentianales
JoTT NOTE 3(9): 2100–2103 New distributional record of Gentiana meadows of the Valley of Flowers tetrasepala Biswas (Gentianales: National Park (VoFNP) (Image Gentianaceae) from the Valley of 1), we report here the recollection Flowers National Park, Garhwal of Gentiana tetrasepala Biswas Himalaya along with the causes of recent threats and the high need of conservation. C.S. Rana 1, V. Rana 2 & M.P.S. Bisht 3 Gentiana tetrasepala was described by Biswas in 1938 on the basis of specimens collected by J.F. 1 State Medicinal Plants Board Uttarakhand, Dehradun, Uttarakhand 248006, India, Duthie (No. 3166 CAL) from Ralam Valley (Kumaon 2,3 Department of Geology, HNB Garhwal University, Srinagar Garhwal) on 26 August 1884. Since then the species (Garhwal), Uttarakhand 246174, India Email: 1 [email protected] (corresponding author), was never recorded leading to the general assumption 2 [email protected], 3 [email protected] that the species had either become extinct or is not a distinct and taxonomically valid species (Garg 1987). Chowdhery & Murti (2000) mentioned this Endemic plants are more prone to extinction for species among the red taxa as per IUCN’s criteria of various reasons as they are habitat specific. Because red taxa (IUCN 1994). It was placed under the ‘IK’ of unstable habitats, in a small area with a limited (insufficiently known) category as per the Indian population they are extra stressed. Therefore, such Red Data Book (Nayar & Shastry 1987). Rawat endemics must be prioritized for conservation efforts (2009) suggested that it be placed under the category (Rawat 2009). Considering this, we have been trying ‘I’ (indeterminate). -

Durham E-Theses
Durham E-Theses Experimental taxonomy of some members of the Teesdale ora Elkington, Trevor Thomas How to cite: Elkington, Trevor Thomas (1962) Experimental taxonomy of some members of the Teesdale ora, Durham theses, Durham University. Available at Durham E-Theses Online: http://etheses.dur.ac.uk/9314/ Use policy The full-text may be used and/or reproduced, and given to third parties in any format or medium, without prior permission or charge, for personal research or study, educational, or not-for-prot purposes provided that: • a full bibliographic reference is made to the original source • a link is made to the metadata record in Durham E-Theses • the full-text is not changed in any way The full-text must not be sold in any format or medium without the formal permission of the copyright holders. Please consult the full Durham E-Theses policy for further details. Academic Support Oce, Durham University, University Oce, Old Elvet, Durham DH1 3HP e-mail: [email protected] Tel: +44 0191 334 6107 http://etheses.dur.ac.uk EXPERIMENTAL TAXONOMY OF SOME MEMBERS OF THE TEESDALE FLORA. BY TREVOR THOMAS ELKINGTON B.Sc. (DUNELM) Being a thesis presented in candidature for the Degree of Doctor of Philosophy in the University of Durham 1962. ACKNOWLEDGEMENTS. I wish to thank Professor D.H. Valentine for suggesting that the Teesdale flora would be a rewarding field for study and for the interest he has taken in supervising my work. I should also like to thank D. Briggs and M.J, Harvey for their stimulating thoughts, and also for their help and that of many other friends and colleagues in collecting material and for giving other help to me. -

Microlepidoptera.Hu Redigit: Fazekas Imre
Microlepidoptera.hu Redigit: Fazekas Imre 5 2012 Microlepidoptera.hu A magyar Microlepidoptera kutatások hírei Hungarian Microlepidoptera News A journal focussed on Hungarian Microlepidopterology Kiadó—Publisher: Regiograf Intézet – Regiograf Institute Szerkesztő – Editor: Fazekas Imre, e‐mail: [email protected] Társszerkesztők – Co‐editors: Pastorális Gábor, e‐mail: [email protected]; Szeőke Kálmán, e‐mail: [email protected] HU ISSN 2062–6738 Microlepidoptera.hu 5: 1–146. http://www.microlepidoptera.hu 2012.12.20. Tartalom – Contents Elterjedés, biológia, Magyarország – Distribution, biology, Hungary Buschmann F.: Kiegészítő adatok Magyarország Zygaenidae faunájához – Additional data Zygaenidae fauna of Hungary (Lepidoptera: Zygaenidae) ............................... 3–7 Buschmann F.: Két új Tineidae faj Magyarországról – Two new Tineidae from Hungary (Lepidoptera: Tineidae) ......................................................... 9–12 Buschmann F.: Új adatok az Asalebria geminella (Eversmann, 1844) magyarországi előfordulásához – New data Asalebria geminella (Eversmann, 1844) the occurrence of Hungary (Lepidoptera: Pyralidae, Phycitinae) .................................................................................................. 13–18 Fazekas I.: Adatok Magyarország Pterophoridae faunájának ismeretéhez (12.) Capperia, Gillmeria és Stenoptila fajok új adatai – Data to knowledge of Hungary Pterophoridae Fauna, No. 12. New occurrence of Capperia, Gillmeria and Stenoptilia species (Lepidoptera: Pterophoridae) ………………………. -

Gentiana Verna, at Home and Abroad by Robert Rolfe------13
ALPINE GARDEN SOCIETY Dublin Group NEWSLETTER NO. 53 – WINTER 2010 CONTENTS Editorial------------------------------------------------------------------------------3 Alpine Miscellany------------------------------------------------------------------4 Philip Shuttleworth by Billy Moore-------------------------------------------11 Gentiana verna, at home and abroad by Robert Rolfe------------------13 Shortias by Liam Byrne----------------------------------------------------------19 Review of Recent Group Events----------------------------------------------22 Fixtures------------------------------------------------------------------------------40 Officers and Committee---------------------------------------------------------42 Front cover illustration is of Gentiana verna (Photo: Liam McCaughey) – see p. 4 2 EDITORIAL A common concern among gardening and horticultural societies in recent years has been the steady and seemingly inexorable fall in numbers. We are an exception: our membership has increased slightly over the past three or four years. It is difficult to pinpoint the reasons for this but it seems logical to conclude that our members are happy with what their subscriptions entitle them to, but the poor attendances at our September/October fixtures would seem to suggest that there may be a problem. One of the main benefits of membership is the excellent programme of lectures on offer each year. The programme is carefully constructed to cater for all interests; from beginners to the more experienced. Our lecturers, whether local or -

Table of Contents
® Table of contents STAUDENSAMEN · PERENNIAL SEEDS · GRAINES DE PLANTES VIVACES Production – Breeding – Seed Technology Throughout the seed list on the following pages you will find: Fax Order Form Signs: Pages 5 – 6 NEW! new introduction The Fax Order Form as well as the with picture in the catalogue Purchase Order Form can be downloaded on www.jelitto.com, Examples out of our descriptions: see „Other“. Order Form Fax ISU = Awarded Plant JEL Jelitto Cultivars and New Introductions, AAS All American Selection, AGM Award of Garden Merit by RHS, FS Fleuro Select, ISU International Hardy Plant Union, PPA Perennial Plant Association, PS Plant Select, contact Plant Select for legal use of ® names UGGET ® N S D E L E 70 cm = height of plants O D G ® · · VI-IX = flowering June - September ® G JELITTO GOLD NUGGET SEED ® O ® JET = JELITTO SEED TECHNOLOGY L D D E N E Pages 8 – 12 represents our improvement in seed cleaning. UGGET S ® JET seeds have been rubbed, peeled or processed into pure seed for List of all available GOLD JELITTO better mechanical handling and seed sowing. NUGGET SEED with important notes. ® The JET process enhances germination on a growing number of GOLD NUGGET SEED seed items. [ ] = These specifications concern cultivar translations, the renaming of existing cultivars, and confusing marketing names given to the botanical species. We deliver the usual trade quality of seed. Perennial Herbs - Culinary, While these specifications are not complete, they are compiled in good Teas and Medicinal conscience to the best of our knowledge and will be updated online (www.jelitto.com). -

Recovery Plan
Approved Recovery Plan Recovery Plan for the “lost” threatened flora of south-eastern NSW Baeuerlen’s Gentian (Gentiana baeuerlenii), Elusive Bush-pea (Pultenaea parrisiae subsp. elusa), Elusive Cress (Irenepharsus magicus), Formbe Peppercress (Lepidium pseudopapillosum), Hidden Violet (Viola cleistogamoides), Mueller’s Eyebright (Euphrasia collina subsp. muelleri), Rosella Spider Orchid (Caladenia rosella), and Swamp Groundsel (Senecio squarrosus) NSW NATIONAL PARKS AND WILDLIFE July 2001 SERVICE © NSW National Parks and Wildlife Service, 2001. This work is copyright. However, material printed in this plan may be copied for personal use or published for educational purposes, provided that any extracts are fully acknowledged. Apart from any use as permitted under the Copyright Act 1968, no part may be reproduced without prior written permission from NPWS. NSW National Parks and Wildlife Service 43 Bridge Street (PO Box 1967) Hurstville NSW 2220 Tel: 02 95856444 www.npws.nsw.gov.au For further information contact Threatened Species Unit, Southern Directorate NSW National Parks and Wildlife Service PO Box 2115 Queanbeyan NSW 2620 Tel (02) 629 89700 Cover photo: Euphrasia collina subsp. paludosa, a species similar in appearance to Mueller’s Eyebright Photographer: Colin Totterdell This plan should be cited as: NSW National Parks and Wildlife Service (2001) Approved Recovery Plan for the “lost” threatened flora of south-eastern NSW. NSW NPWS, Hurstville, NSW. ISBN: 0 7313 6205 5 Approved Recovery Plan Lost threatened flora of SE NSW Recovery Plan for the “lost” threatened flora of south-eastern NSW: Baeuerlen’s Gentian (Gentiana baeuerlenii), Elusive Bush-pea (Pultenaea parrisiae subsp. elusa), Elusive Cress (Irenepharsus magicus), Formbe Peppercress (Lepidium pseudopapillosum), Hidden Violet (Viola cleistogamoides), Mueller’s Eyebright (Euphrasia collina subsp.