Ambroxol-Bromhexine (EMEA-H-A-31-1397)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

More Than Expectorant: New Scientific Data on Ambroxol in the Context of the Treatment of Bronchopulmonary Diseases
Mini Review iMedPub Journals Journal of Intensive and Critical Care 2017 www.imedpub.com ISSN 2471-8505 Vol. 3 No. 3: 37 DOI: 10.21767/2471-8505.100096 More than Expectorant: New Scientific Data Manuel Plomer1* and 2 on Ambroxol in the Context of the Treatment Justus de Zeeuw of Bronchopulmonary Diseases 1 Medical Affairs CHC Germany, Sanofi-Aventis Deutschland GmbH, Industriepark Höchst, D-65926, Frankfurt am Main, Deutschland, German Abstract 2 Medical Specialist for Internal Medicine, Pneumology and Sleep Medicine, Background:Ambroxol has been established for decades in the treatment of Cologne, Germany, German acute and chronic respiratory diseases. A reassessment of the benefit-risk was conducted recently. Objective: What new scientific data, relevant for the treatment of *Corresponding author: bronchopulmonary diseases, were published in the last decade? Dr. Manuel Plomer Method: Systematic literature search via http://www.pubmed.gov with the search term “ambroxol”, covering the publication period from 2006 to 2015. Non- [email protected] relevant publications were excluded manually. Medical Manager, Medical Affairs CHC Results: 64 relevant publications could be identified covering both, clinical and Germany, Sanofi-Aventis Deutschland GmbH, preclinical research. Industriepark Höchst, D-65926 Frankfurt am Conclusion: The traditional indication of ambroxol as an expectorant is confirmed Main, Germany. but new results revealed a better understanding of the various mechanisms of action of ambroxol and the benefits for special patient populations. The Tel: 49 69 305 36803 available data suggest the use of ambroxol as an adjuvant in anti-infective therapy, particularly in case of infections with biofilm-producing pathogens. Lung- protective properties are discussed in both infants and severely ill adult patients. -

European Journal of Scientific Exploration Vol 3 №2 2020
EUROPEAN JOURNAL OF SCIENTIFIC EXPLORATION VOL 3 №2 2020 Determination of Oligomer Content in Benzonatate Drug Substance by HPLC Lakshmi Narasimha Rao Katakam 1 Santhosh Kumar Ettaboina 2 Thirupathi Dongala 2 1Saptalis Pharmaceuticals LLC, New York, USA 2Aurex Pharmaceuticals Inc, East Windsor, USA Abstract. A reverse-phase liquid chromatographic method has developed and validated to determine oligomer content in Benzonatate Drug substances. This method has a separation of relatively few monomer units, which constitutes an oligomer with a minimum adequate chromatographic resolution of 1.0 from each of the subject component peaks. The separation achieved using Phenomenex Luna C18 (250 X 4.6 mm) 5 µm column at a flow rate of 1.0 ml/min with an isocratic elution method. The mobile phase consisting of 0.25% Ammonium formate buffer and methanol in the ratio 350:650 (v/v), respectively. The Oligomer compounds detection carried out at UV 310 nm, and the LC method validated as per the current ICH Q2 guidelines. The method is effectively validated and proved to be precise, specific, linear, robust, and rugged to quantitate oligomer content in Benzonatate drug substance. Key words: benzonatate (BNZ), oligomers, HPLC, method validation. Introduction Benzonatate (BNZ) is an oral antitussive drug used to relieve and suppress cough in patients older than ten years of age (Oligomer Wikipedia, 2020). Currently, BNZ is the only non-narcotic antitussive available as a prescription drug. The chemical structure resembles that of the anesthetic agents in the para-amino-benzoic acid class (such as procaine and tetracaine), BNZ exhibits anesthetic or numbing action. BNZ also inhibits the transmission of impulses of the cough reflex in the medulla's vagal nuclei (Tessalon, 2020). -

Appendix on Tariff Elimination Schedule for Mercosur
Trade part of the EU-Mercosur Association Agreement Without Prejudice Disclaimer: In view of the Commission's transparency policy, the Commission is publishing the texts of the Trade Part of the Agreement following the agreement in principle announced on 28 June 2019. The texts are published for information purposes only and may undergo further modifications including as a result of the process of legal revision. However, in view of the growing public interest in the negotiations, the texts are published at this stage of the negotiations for information purposes. These texts are without prejudice to the final outcome of the agreement between the EU and Mercosur. The texts will be final upon signature. The agreement will become binding on the Parties under international law only after completion by each Party of its internal legal procedures necessary for the entry into force of the Agreement (or its provisional application). AR applied BR applied PY applied UY applied Mercosur Final NCM Description Comments tariff tariff tariff tariff Offer 01012100 Pure-bred horses 0 0 0 0 0 01012900 Lives horses, except pure-bred breeding 2 2 2 2 0 01013000 Asses, pure-bred breeding 4 4 4 4 4 01019000 Asses, except pure-bred breeding 4 4 4 4 4 01022110 Purebred breeding cattle, pregnant or lactating 0 0 0 0 0 01022190 Other pure-bred cattle, for breeding 0 0 0 0 0 01022911 Other bovine animals for breeding,pregnant or lactating 2 2 2 2 0 01022919 Other bovine animals for breeding 2 2 2 2 4 01022990 Other live catlle 2 2 2 2 0 01023110 Pure-bred breeding buffalo, pregnant or lactating 0 0 0 0 0 01023190 Other pure-bred breeding buffalo 0 0 0 0 0 01023911 Other buffalo for breeding, ex. -

Antitussive Effect of a Fixed Combination of Justicia Adhatoda
Phytomedicine 22 (2015) 1195–1200 Contents lists available at ScienceDirect Phytomedicine journal homepage: www.elsevier.com/locate/phymed Antitussive effect of a fixed combination of Justicia adhatoda, Echinacea purpurea and Eleutherococcus senticosus extracts in patients with acute upper respiratory tract infection: A comparative, randomized, double-blind, placebo-controlled study Anders Barth b, Areg Hovhannisyan c,∗, Kristina Jamalyan a, Mikael Narimanyan a a Yerevan State Medical University of Armenia, Koryun 2 0025, Yerevan, Armenia b Partus Kvinnohälsa, Södra v. 2, 412 54, Göteborg c Anti-doping Service of Republican Centre of Sport Medicine, Acharyan Street, 2/6, Yerevan, Armenia article info abstract Article history: Background: Kan Jang® oral solution (KJ) is a fixed combination of aqueous ethanolic extracts of Justicia Received 20 August 2015 adhatoda L. leaf,Echinaceapurpurea(L.) Moench root, and Eleutherococcus senticosus (Rupr. & Maxim.) Harms Revised 2 October 2015 root. It is approved in Scandinavia as an herbal medicinal product for respiratory tract infection treatment. Accepted 4 October 2015 Purpose: The present clinical trial aimed to compare the antitussive effect of KJ with placebo (PL) and bromhexine (BH) among patients of 18–65 years old with non-complicated upper respiratory infections (URI; Keywords: i.e., common cold). Cough Study design: We performed a parallel-group, randomized, double-blinded, placebo-controlled trial in in 177 Kan Jang patients with acute URI over a 5 day period. Randomized controlled trial Methods: We investigated the antitussive effects of a KJ (30 ml/day; 762 mg genuine extracts with stan- dardized contents of 0.2 mg/ml vasicine, 0.8 mg/ml chicoric acid, and 0.03 mg/ml eleutherosides B and E), bromhexine hydrochloride (24 mg/30 ml/day) and PL on cough and blood markers. -

Research Article
Volume 8, Issue 2, May – June 2011; Article-004 ISSN 0976 – 044X Research Article SPECTROPHOTOMETRIC SIMULTANEOUS ANALYSIS OF AMBROXOL HYDROCHLORIDE, GUAIFENESIN AND TERBUTALINE SULPHATE IN LIQUID DOSAGE FORM (SYRUP) Ritu Kimbahune*, Sunil K, Prachi Kabra, Kuldeep Delvadiya, Sanjay Surani Department of Quality Assurance, Nargund College of Pharmacy, Dattatreyanagar, Bangalore - 560 085, India. Accepted on: 06-03-2011; Finalized on: 28-05-2011. ABSTRACT This study proposes a method for simultaneous estimation of Ambroxol HCl, Guaifenesin and Terbutaline sulphate in syrup form. The study was done by combining three spectrophotometric methods viz use of specific absorbance [A 1%, 1cm], second order derivative and colorimetry. Absorption of Guaifenesin and Terbutaline sulphate were found to be zero at 307.5nm, thus enabling the measurement of Ambroxol HCl, using specific absorbance in zero order spectrum. Applying the second order derivative, the amplitude of Guaifenesin was measured at 279.4nm, while Ambroxol HCl and Terbutaline sulphate were at zero cross point. For colorimetric measurement of Terbutaline Sulphate, a colored substance was obtained by coupling the oxidized product of Terbutaline sulphate with 4-aminoantipyrine and its absorption was measured at 550nm. The proposed method was statistically validated in accordance with ICH guidelines and results were found to be satisfactory for accuracy, precision and specificity. Keywords: Ambroxol HCl, Guaifenesin and Terbutaline sulphate, second order derivative, colorimetric method. INTRODUCTION TBS as an individual drug or in combination, either in pure or in pharmaceutical forms as well as in biological Ambroxol hydrochloride (AB) [trans-4-[(2-amino-3,5- fluids and tissues. Literature survey reveals that there is dibromobenzyl)amino]cyclohexanol hydrochloride] is a no single spectrophotometric analysis reported for the semi-synthetic derivative of vasicine obtained from Indian determination of AB, GF and TBS simultaneously in liquid shrub Adhatodavasica. -
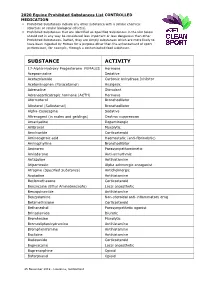
2020 Equine Prohibited Substances List CONTROLLED MEDICATION
2020 Equine Prohibited Substances List CONTROLLED MEDICATION . Prohibited Substances include any other substance with a similar chemical structure or similar biological effect(s). Prohibited Substances that are identified as Specified Substances in the List below should not in any way be considered less important or less dangerous than other Prohibited Substances. Rather, they are simply substances which are more likely to have been ingested by Horses for a purpose other than the enhancement of sport performance, for example, through a contaminated food substance. SUBSTANCE ACTIVITY 17-Alpha-Hydroxy Progesterone FEMALES Hormone Acepromazine Sedative Acetazolamide Carbonic Anhydrase Inhibitor Acetominophen (Paracetamol) Analgesic Adrenaline Stimulant Adrenocorticotropic hormone (ACTH) Hormone Aformoterol Bronchodilator Albuterol (Salbutamol) Bronchodilator Alpha-Casozepine Sedative Altrenogest (in males and geldings) Oestrus suppression Amantadine Dopaminergic Ambroxol Mucolytic Amcinonide Corticosteroid Aminocaproic acid Haemostatic (anti-fibrinolytic) Aminophylline Bronchodilator Aminorex Parasympathomimetic Amiodarone Anti-arrhythmic Antazoline Antihistamine Atipamezole Alpha adrenergic antagonist Atropine (Specified Substance) Anticholinergic Azatadine Antihistamine Beclomethasone Corticosteroid Benzocaine (Ethyl Aminobenzoate) Local anaesthetic Benzquinamide Antihistamine Benzydamine Non-steroidal anti-inflammatory drug Betamethasone Corticosteroid Bethanechol Parasympathetic agonist Brinzolamide Diuretic Bromhexine Mucolytic Bromodiphenhydramine -

Pharmaceutical Appendix to the Harmonized Tariff Schedule
Harmonized Tariff Schedule of the United States (2019) Revision 13 Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2019) Revision 13 Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names INN which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service CAS registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. -

1. Generic Names Levosalbutamol 0.25G Ambroxol 7.5Mg
1. Generic Names Levosalbutamol 0.25g Ambroxol 7.5mg Guaiphenesin 12.5 mg 2. Qualitative and Quantitative Composition Each ml contains Levosalbutamol 0.25g Ambroxol 7.5mg Guaiphenesin 12.5 mg 3. Dosage form and strength Oral drop solution containing Levosalbutamol 0.25g, Ambroxol 7.5mg, Guaiphenesin 12.5 mg. 4. Clinical particulars 4.1 Therapeutic indication Kofarest-PD drops is indicated for the treatment of productive cough associated with bronchospasm in conditions such as bronchitis and bronchial asthma as well as all conditions associated with tenacious mucus, wheezing and chest congestion. 4.2 Posology and method of administration The usual recommended dose of KOFAREST-PD Drops in children is: 1-2 years age: 0.8 ml thrice a day 2-3 years age: 1.2 ml thrice a day. 4.3 Contraindication Kofarest-PD drops are contraindicated in patients with hypersensitivity to any ingredient of the formulation. 4.4 Special warnings and precautions for use While treating cough as a symptom, it is important to make every effort to determine and treat appropriately the underlying cause, such as a specific infection. Caution should be observed while prescribing Kofarest-PD drops to children with hypertension, cardiovascular disease, uncontrolled juvenile diabetes mellitus, hyperthyroidism, and seizures or in patients who are unusually hypersensitive to sympathomimetic amines. 4.5 Drug interactions Hypokalaemia with high doses of ß2 -agonists may result in increased susceptibility to digitalis induced cardiac arrhythmias. Hypokalaemia may be enhanced by concomitant administration of aminophylline or other xanthine, corticosteroids or by diuretic therapy. Other sympathomimetic bronchodilators or epinephrine should not be used concomitantly with salbutamol, since their combined effect on the cardiovascular system may be deleterious to the patient. -

National OTC Medicines List
National OTC Medicines List ‐ DraŌ 01 DRAFT National OTC Medicines List Draft 01 Ministry of Public Health of Lebanon This list was prepared under the guidance of His Excellency Minister Waêl Abou Faour andDRAFT the supervision of the Director General Dr. Walid Ammar. Editors Rita KARAM, Pharm D. PhD. Myriam WATFA, Pharm D Ghassan HAMADEH, MD.CPE FOREWORD According to the French National Agency for Medicines and Health Products Safety (ANSM), Over-the-counter (OTC) drugs are medicines that are accessible to patients in pharmacies, based on criteria set to safeguard patients’ safety. Due to their therapeutic class, these medicines could be dispensed without physician’s intervention for diagnostic, treatment initiation or maintenance purposes. Moreover, their dosage, treatment period and Package Insert Leaflet should be suitable for OTC classification. The packaging size should be in accordance with the dosage and treatment period. According to ArticleDRAFT 43 of the Law No.367 issued in 1994 related to the pharmacy practice, and the amendment of Articles 46 and 47 by Law No.91 issued in 2010, pharmacists do not have the right to dispense any medicine that is not requested by a unified prescription, unless the medicine is mentioned in a list which is established by pharmacists and physicians’ syndicates. In this regard, the Ministry of Public Health (MoPH) developed the National OTC Medicines List, and presentedit in a scientific, objective, reliable, and accessible listing. The OTC List was developed by a team of pharmacists and physicians from the Ministry of Public Health (MoPH). In order to ensure a safe and effective self- medicationat the pharmacy level, several pharmaceutical categories (e.g. -

Trends in Prescription Medication Use Among Children and Adolescents— United States, 1999-2014
Supplementary Online Content Hales CM, Kit BK, Gu Q, Ogden CL. Trends in prescription medication use among children and adolescents— United States, 1999-2014. JAMA. doi:10.1001/jama.2018.5690 eTable. Classification of Prescription Medications Reported by NHANES Participants Aged 0-19 Years From 1999- 2000 to 2013-2014 by Therapeutic Class This supplementary material was provided by the authors to give readers additional informtion about their work. © 2018 American Medical Association. All rights reserved. Downloaded From: https://jamanetwork.com/ on 09/27/2021 eTable. Classification of Prescription Medications Reported by NHANES Participants Aged 0-19 Years From 1999- 2000 to 2013-2014 by Therapeutic Class Therapeutic classes are based on the Lexicon Plus prescription medication database and only those classes reported in the manuscript are listed. ADHD Medications Antiadrenergic Agents, Centrally Acting Clonidine Guanfacine CNS Stimulants Amphetamines Amphetamine Amphetamine; Dextroamphetamine Dextroamphetamine Lisdexamfetamine Methylphenidate or Dexmethylphenidate Dexmethylphenidate Methylphenidate Other CNS Stimulant Pemoline Selective Norepinephrine Reuptake Inhibitor Atomoxetine Antibiotics Cephalosporins Cefadroxil Cephalexin Cefaclor Cefprozil Cefuroxime Loracarbef Cefdinir Cefditoren Cefixime Cefpodoxime Ceftibuten Ceftriaxone Glycopeptide Antibiotics Vancomycin H. Pylori Eradication Agents Amoxicillin; Clarithromycin; Lansoprazole Lincomycin Derivatives Clindamycin Macrolide Derivatives Telithromycin Azithromycin Clarithromycin -

Mucoactive Agents for Airway Mucus Hypersecretory Diseases
Mucoactive Agents for Airway Mucus Hypersecretory Diseases Duncan F Rogers PhD FIBiol Introduction Sputum Profile of Airway Inflammation and Mucus Hypersecretory Phenotype in Asthma, COPD, and CF Which Aspect of Airway Mucus Hypersecretion to Target? Theoretical Requirements for Effective Therapy of Airway Mucus Hypersecretion Current Recommendations for Clinical Use of Mucolytic Drugs Mucoactive Drugs N-Acetylcysteine: How Does it Work? Does it Work? Dornase Alfa Hypertonic Saline Surfactant Analysis Summary Airway mucus hypersecretion is a feature of a number of severe respiratory diseases, including asthma, chronic obstructive pulmonary disease (COPD), and cystic fibrosis (CF). However, each disease has a different airway inflammatory response, with consequent, and presumably linked, mucus hypersecretory phenotype. Thus, it is possible that optimal treatment of the mucus hyper- secretory element of each disease should be disease-specific. Nevertheless, mucoactive drugs are a longstanding and popular therapeutic option, and numerous compounds (eg, N-acetylcysteine, erdosteine, and ambroxol) are available for clinical use worldwide. However, rational recommen- dation of these drugs in guidelines for management of asthma, COPD, or CF has been hampered by lack of information from well-designed clinical trials. In addition, the mechanism of action of most of these drugs is unknown. Consequently, although it is possible to categorize them according to putative mechanisms of action, as expectorants (aid and/or induce cough), mucolytics (thin -

Bromhexine Hydrochloride
Bromhexine Hydrochloride sc-204656 Material Safety Data Sheet Hazard Alert Code EXTREME HIGH MODERATE LOW Key: Section 1 - CHEMICAL PRODUCT AND COMPANY IDENTIFICATION PRODUCT NAME Bromhexine Hydrochloride STATEMENT OF HAZARDOUS NATURE CONSIDERED A HAZARDOUS SUBSTANCE ACCORDING TO OSHA 29 CFR 1910.1200. NFPA FLAMMABILITY1 HEALTH3 HAZARD INSTABILITY0 SUPPLIER Company: Santa Cruz Biotechnology, Inc. Address: 2145 Delaware Ave Santa Cruz, CA 95060 Telephone: 800.457.3801 or 831.457.3800 Emergency Tel: CHEMWATCH: From within the US and Canada: 877-715-9305 Emergency Tel: From outside the US and Canada: +800 2436 2255 (1-800-CHEMCALL) or call +613 9573 3112 PRODUCT USE Expectorant. Reported to change the structure of bronchial secretions and to increase the volume and reduce the viscosity of sputum. Administered in bronchitis and other respiratory conditions. Normally given by mouth. Synthetic derivative of vasicine, the active principal of Adhatoda vasica. SYNONYMS C14-H20-Br2-N2.HCl, "2-amino-N-cyclohexyl-3, 5-dibromo-N-methylbenzylamine hydrochloride", "2-amino-N-cyclohexyl-3, 5-dibromo-N-methylbenzylamine hydrochloride", "benzenemethanamine, 2-amino-3, 5-dibromo-N-cyclohexyl-N-methyl-, ", "benzenemethanamine, 2-amino-3, 5-dibromo-N-cyclohexyl-N-methyl-, ", monohydrochloride, "benzylamine, 2-amino-N- cyclohexyl-3, 5-dibromo-N-methyl-, hydrochloride", "benzylamine, 2-amino-N-cyclohexyl-3, 5-dibromo-N-methyl-, hydrochloride", "bromhexin hydrochloride", "N-cyclohexyl-N-methyl-(2-amino-3, 5-dibromobenzyl) ammonium chloride", "N- cyclohexyl-N-methyl-(2-amino-3, 5-dibromobenzyl) ammonium chloride", Aletor, Bisolvomycin, Bisolvon, "Bisolvon Hydrochloride", Bromessina, Bromcilate, Bronkocin, "Dakroy Biciron", Ophtosol, "quaternary ammonium expectorant" Section 2 - HAZARDS IDENTIFICATION CANADIAN WHMIS SYMBOLS EMERGENCY OVERVIEW RISK Harmful if swallowed.