Annex VI List of Chemical and Biological Items, Materials
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Effect of Diethylenetriamine and Triethylamine Sensitization on the Critical Diameter of Nitromethane’
CP505, Shock Compression of Condensed Matter - 1999 edited by M. D. Furnish, L. C. Chhabildas, and R. S. Hixson 0 2000 American Institute of Physics l-56396-923-8/00/$17.00 EFFECT OF DIETHYLENETRIAMINE AND TRIETHYLAMINE SENSITIZATION ON THE CRITICAL DIAMETER OF NITROMETHANE’ J.J. Lee*, J. Jiang?, K.H. Choong’, J.H.S. Lee’ *Graduate Aeronautics Laboratory, California Institute of Technology, Pasadena, CA 9112.5, USA ‘Dept. of Mechanical Engineering, McGill University, Montr&al, Que’bec, Canada, H3A 2K6 In this work, the critical diameter for detonation was measured for Nitromethane (NM) sensitized with two different amines: Diethylenetriamine (DETA) and Triethylamine (TEA). The critical diameter in glass and polyvinylchloride tubes is found to decrease rapidly as the amount of sensitizer is increased, then increase past a critical amount of sensitizer. Thus the critical diameter reaches a minimum at a critical concentration of sensitizer. It was also found that the critical diameter is lower with DETA than with TEA. INTRODUCTION propagation in various tubes and channels, and the critical conditions for propagation in porous media Previous studies have shown that small (3) . concentrations of certain substances can strongly The effect of DETA on the critical diameter of increase the explosive sensitivity nitromethane NM has been reported by Engelke (4), who (NM). Amines are found to be the most effective performed measurements with up to 2.5% DETA by chemical sensitizing agent for NM with mass in NM. Engelke observed a reduction in the ethylenediamine and diethylenetriamine (DETA) critical diameter of over 50% in the range of DETA producing the largest increase in the card gap value concentrations used. -

Theoretical Study of Sarin Adsorption On
Chemical Physics Letters 738 (2020) 136816 Contents lists available at ScienceDirect Chemical Physics Letters journal homepage: www.elsevier.com/locate/cplett Research paper Theoretical study of sarin adsorption on (12,0) boron nitride nanotube doped with silicon atoms T ⁎ ⁎ Jeziel Rodrigues dos Santosa, , Elson Longo da Silvab, Osmair Vital de Oliveirac, , José Divino dos Santosa a Universidade Estadual de Goiás, Campus Anápolis, CEP: 75.132-903 GO, Brazil b INCTMN, LIEC, Departamento de Química da Universidade Federal de São Carlos, CEP: 13.565-905 São Carlos, SP, Brazil c Instituto Federal de Educação, Ciência e Tecnologia de São Paulo, Campus Catanduva, CEP: 15.808-305 Catanduva, SP, Brazil HIGHLIGHTS • DFT method was used to study the adsorption of nerve agent sarin by BNNT. • Electronic properties of pristine BNNT are improved by Si impurity atoms. • The adsorption of sarin by Si-doped BNNT is highest favorable than the pure BNNT. • Si-doped BNNT can be a new gas sensor for sarin gas detection and its derivatives. ARTICLE INFO ABSTRACT Keywords: Sarin gas is one of the most lethal nerve agent used in chemical warfare, which its detection is import to prevent Nerve agent sarin a chemical attack and to identify a contamination area. Herein, density functional theory was used to investigate Gas sensor the (12,0) boron nitride nanotube (BNNT) and Si–doped BNNT as possible candidates to sarin detection. The Si- Boron nitride nanotube atoms doped improve the electronic properties of nanotubes by altering the electrostatic potential, HOMO and DFT LUMO energies. Based in the adsorption energies and the conductivity increased to ~33 and 350%, respectively, for Si- and 2Si-BNNT imply that they can be used for sarin detection. -

Industry Compliance Programme
Global Chemical Industry Compliance Programme GC-ICP Chemical Weapons Convention December 2006 Version 1.0 GLOBAL CHEMICAL INDUSTRY COMPLIANCE PROGRAMME FOR IMPLEMENTING THE CHEMICAL WEAPONS CONVENTION The purpose of the handbook is to provide guidance to chemical facilities, traders and trading companies in developing a Global Chemical Industry Compliance Programme (GC-ICP) to comply with the Chemical Weapons Convention (CWC). The GC-ICP focuses first on determining if there is a reporting requirement to your National Authority and second on collecting the relevant support data used to complete the required reports. The GC-ICP is designed to provide a methodology to comply with the CWC and establish systems that facilitate and demonstrate such compliance. Each facility/company should also ensure that it follows its country’s CWC specific laws, regulations and reporting requirements. • Sections 2, 3, and 4 guide you through the process of determining if chemicals at your facility/ company should be reported to your National Authority for compliance with the CWC. • Section 5 provides recommended guidance on information that you may use to determine your reporting requirements under the CWC and administrative tools that your facility/company may use to ensure compliance with the CWC. • Section 6 provides a glossary of terms and associated acronyms. • Section 7 provides a listing of all National Authorities by country. CWC Global Chemical Industry Compliance Programme 1 TABLE OF CONTENTS Section 1 Overview What is the Chemical Weapons Convention? -

A Quantum Chemical Study Involving Nitrogen Mustards
The Pharmaceutical and Chemical Journal, 2016, 3(4):58-60 Available online www.tpcj.org ISSN: 2349-7092 Research Article CODEN(USA): PCJHBA Formation enthalpy and number of conformers as suitable QSAR descriptors: a quantum chemical study involving nitrogen mustards Robson Fernandes de Farias Universidade Federal do Rio Grande do Norte, Cx. Postal 1664, 59078-970, Natal-RN, Brasil Abstract In the present work, a quantum chemical study (Semi-empirical,PM6 method) is performed using nitrogen mustards (HN1, HN2 and HN3) as subjects in order to demonstrate that there is a close relationship between pharmacological activity and parameters such as formation enthalpy and number of conformers, which could, consequently, be employed as reliable QSAR descriptors. To the studied nitrogen mustards, a very simple equation o o relating log P, ΔH f and the number of conformers (Nc) was found: log P = [(log -ΔH f + logNc)/2]-0.28. Keywords QSAR, Descriptors, Formation enthalpy, Conformers, Semi-empirical, Nitrogen mustards, Log P Introduction It is well known that lipophilicity is a very important molecular descriptor that often correlates well with the bioactivity of chemicals [1]. Hence, lipophilicity, measured as log P, is a key property in quantitative structure activity relationship (QSAR) studies. In this connection, in the pharmaceutical sciences it is a common practice to use log P (the partition coefficient between water and octanol), as a reliable indicator of the hydrophobicity or lipophilicity of (drug) molecules [1-2]. For example, relying primarily on the log P is a sensible strategy in preparing future 18-crown-6 analogs with optimized biological activity [3]. -

General Listing Background Document for the Inorganic Chemical Listing Determination
GENERAL LISTING BACKGROUND DOCUMENT FOR THE INORGANIC CHEMICAL LISTING DETERMINATION August, 2000 U.S. ENVIRONMENTAL PROTECTION AGENCY ARIEL RIOS BUILDING 1200 PENNSYLVANIA AVENUE, N.W. WASHINGTON, D.C. 20460 TABLE OF CONTENTS Page LIST OF TABLES .............................................................ii LIST OF FIGURES ............................................................ii LIST OF APPENDICES .........................................................ii 1. INTRODUCTION .......................................................1 1.1 BACKGROUND ...................................................1 1.2 EXISTING INORGANIC CHEMICAL LISTINGS ........................2 1.3 OTHER EPA REGULATORY PROGRAMS AFFECTING THE INORGANIC CHEMICAL INDUSTRY ............................................3 2. INDUSTRY DESCRIPTION .........................................5 2.1 INDUSTRY PROFILE ..............................................5 2.2 INDUSTRY SECTORS .............................................5 2.2.1 Antimony Oxide ..............................................8 2.2.2 Barium Carbonate ............................................8 2.2.3 Boric Acid ..................................................8 2.2.4 Cadmium Pigments ............................................8 2.2.5 Inorganic Hydrogen Cyanide ....................................8 2.2.6 Phenyl Mercuric Acetate .......................................8 2.2.7 Dry Process Phosphoric Acid ....................................8 2.2.8 Phosphorous Pentasulfide .......................................8 -
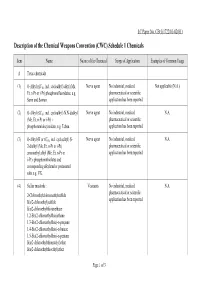
Description of the Chemical Weapons Convention (CWC) Schedule 1 Chemicals
LC Paper No. CB(1)1722/01-02(01) Description of the Chemical Weapons Convention (CWC) Schedule 1 Chemicals Item Name Nature of the Chemical Scope of Application Examples of Common Usage A Toxic chemicals (1) O-Alkyl (≤C10, incl. cycloalkyl) alkyl (Me, Nerve agent No industrial, medical, Not applicable (N.A.) Et, n-Pr or i-Pr) phosphonofluoridates, e.g. pharmaceutical or scientific Sarin and Soman. application has been reported. (2) O-Alkyl (≤C10, incl. cycloalkyl) N,N-dialkyl Nerve agent No industrial, medical, N.A. (Me, Et, n-Pr or i-Pr) - pharmaceutical or scientific phosphoramidocyanidate, e.g. Tabun. application has been reported. (3) O-Alkyl (H or ≤C10, incl. cycloalkyl) S- Nerve agent No industrial, medical, N.A. 2-dialkyl (Me, Et, n-Pr or i-Pr) pharmaceutical or scientific aminoethyl alkyl (Me, Et, n-Pr or application has been reported. i-Pr)- phosphonothiolates and corresponding alkylated or protonated salts e.g. VX. (4) Sulfur mustards : Vesicants No industrial, medical, N.A. pharmaceutical or scientific 2-Chloroethylchloromethylsulfide application has been reported. Bis(2-chloroethyl)sulfide Bis(2-chloroethylthio)methane 1,2-Bis(2-chloroethylthio)ethane 1,3-Bis(2-chloroethylthio)-n-propane 1,4-Bis(2-chloroethylthio)-n-butane 1,5-Bis(2-chloroethylthio)-n-pentane Bis(2-chloroethylthiomethyl)ether Bis(2-chloroethylthioethyl)ether Page 1 of 3 Item Name Nature of the Chemical Scope of Application Examples of Common Usage (5) Lewisites : Vesicants No industrial, medical, N.A. pharmaceutical or scientific Lewisite 1 : 2-Chlorovinyldichloroarsine application has been reported. Lewisite 2 : Bis(2-chlorovinyl)chloroarsine Lewisite 3 : Tris(2-chlorovinyl)arsine (6) Nitrogen mustards : Vesicants The chemical has medical Only HN2 has been reported to application. -

779 Part 770—Interpretations
Pt. 770 15 CFR Ch. VII (1–1–21 Edition) in the item that qualitatively affect the per- roller bearings and parts). This applies formance of the U.S. and foreign items; to separate shipments of anti-friction (vi) Evidence of the interchangeability of bearings or bearing systems and anti- U.S. and foreign items; friction bearings or bearing systems (vii) Patent descriptions for the U.S. and foreign items; shipped with machinery or equipment (viii) Evidence that the U.S. and foreign for which they are intended to be used items meet a published industry, national, or as spares or replacement parts. international standard; (2) An anti-friction bearing or bear- (ix) A report or eyewitness account, by ing system physically incorporated in a deposition or otherwise, of the foreign item’s segment of a machine or in a complete operation; machine prior to shipment loses its (x) Evidence concerning the foreign manu- identity as a bearing. In this scenario, facturers’ corporate reputation; (xi) Comparison of the U.S. and foreign end the machine or segment of machinery item(s) made from a specific commodity, containing the bearing is the item sub- tool(s), device(s), or technical data; or ject to export control requirements. (xii) Evidence of the reputation of the for- (3) An anti-friction bearing or bear- eign item including, if possible, information ing system not incorporated in a seg- on maintenance, repair, performance, and ment of a machine prior to shipment, other pertinent factors. but shipped as a component of a com- plete unassembled (knocked-down) ma- SUPPLEMENT NO. -

Toxicological Profile for Glyphosate Were
A f Toxicological Profile for Glyphosate August 2020 GLYPHOSATE II DISCLAIMER Use of trade names is for identification only and does not imply endorsement by the Agency for Toxic Substances and Disease Registry, the Public Health Service, or the U.S. Department of Health and Human Services. GLYPHOSATE III FOREWORD This toxicological profile is prepared in accordance with guidelines developed by the Agency for Toxic Substances and Disease Registry (ATSDR) and the Environmental Protection Agency (EPA). The original guidelines were published in the Federal Register on April 17, 1987. Each profile will be revised and republished as necessary. The ATSDR toxicological profile succinctly characterizes the toxicologic and adverse health effects information for these toxic substances described therein. Each peer-reviewed profile identifies and reviews the key literature that describes a substance's toxicologic properties. Other pertinent literature is also presented, but is described in less detail than the key studies. The profile is not intended to be an exhaustive document; however, more comprehensive sources of specialty information are referenced. The focus of the profiles is on health and toxicologic information; therefore, each toxicological profile begins with a relevance to public health discussion which would allow a public health professional to make a real-time determination of whether the presence of a particular substance in the environment poses a potential threat to human health. The adequacy of information to determine a substance's -

"The Science for Diplomats" Annex on Chemicals
ORGANISATION FOR THE PROHIBITION OF CHEMICAL WEAPONS "THE SCIENCE FOR DIPLOMATS" ANNEX ON CHEMICALS A user friendly and scientifically annotated version of the Chemical Weapons Convention Annex on Chemicals OPCW THE “SCIENCE FOR DIPLOMATS” ANNEX ON CHEMICALS A user friendly and scientifically annotated version of the Chemical Weapons Convention Annex on Chemicals1 CONTENTS A. GUIDELINES FOR SCHEDULES OF CHEMICALS B. VISUALISING AND READING MOLECULAR STRUCTURES C. SCHEDULES OF CHEMICALS D. RIOT CONTROL AGENTS 1 An official version of the Annex on Chemicals can be obtained from the OPCW public website, www.opcw.org/chemical-weapons-convention/annexes/annex-chemicals/annex-chemicals. Version 3.0 – 10 March 2019 A. GUIDELINES FOR SCHEDULES OF CHEMICALS Guidelines for Schedule 1 1. The following criteria shall be taken into account in considering whether a toxic chemical or precursor should be included in Schedule 1: (a) It has been developed, produced, stockpiled or used as a chemical weapon as defined in Article II; (b) It poses otherwise a high risk to the object and purpose of this Convention by virtue of its high potential for use in activities prohibited under this Convention because one or more of the following conditions are met: (i) It possesses a chemical structure closely related to that of other toxic chemicals listed in Schedule 1, and has, or can be expected to have, comparable properties; (ii) It possesses such lethal or incapacitating toxicity as well as other properties that would enable it to be used as a chemical weapon; (iii) It may be used as a precursor in the final single technological stage of production of a toxic chemical listed in Schedule 1, regardless of whether this stage takes place in facilities, in munitions or elsewhere; (c) It has little or no use for purposes not prohibited under this Convention. -

Trihalomethanes/MTBE/Nitromethane Lab Procedure Manual
Laboratory Procedure Manual Analyte: Trihalomethanes/MTBE/Nitromethane Matrix: Whole Blood Method: Solid Phase Microextraction with GC Separation/High Resolution MS Method No: 2101.01 Revised: April 30, 2015 As performed by: Tobacco & Volatiles Branch Division of Laboratory Sciences National Center for Environmental Health Contact: Dr. Ben Blount Phone: 770-488-7894 Fax: 770-488-0181 Email: [email protected] James L. Pirkle, M.D., Ph.D. Director, Division of Laboratory Sciences Important Information for Users The Centers for Disease Control and Prevention (CDC) periodically refines these laboratory methods. It is the responsibility of the user to contact the person listed on the title page of each write-up before using the analytical method to find out whether any changes have been made and what revisions, if any, have been incorporated. THMs & MTBE VOCs in Blood DLS Method Code: 2101.01 National Center for Health Staistics 2 This document details the Lab Protocol for testing the items listed in the following table Data File Name Variable Name SAS Label LBXVBF Blood Bromoform (pg/mL) LBXVBM Blood Bromodichloromethane (pg/mL) VOCMWB_F LBXVCF Blood Chloroform (pg/mL) LBXVCM Blood Dibromochloromethane (pg/mL) LBXVME Blood MTBE (pg/mL) LBXVNM Blood Nitromethane (pg/mL) THMs & MTBE VOCs in Blood DLS Method Code: 2101.01 National Center for Health Staistics 3 1. Clinical Relevance and Summary of Test Principle a. Clinical Relevance The prevalence of disinfection by-products in drinking water supplies has raised concerns about possible adverse health effects from chronic exposure to these potentially carcinogenic compounds. To support studies exploring the relation between exposure to trihalomethanes (THMs), nitromethane (NM: biomarker for halonitromethanes), methyl tert-butyl ether (MTBE) and adverse health effects, an automated analytical method was developed using capillary gas chromatography (GC) and high-resolution mass spectrometry (MS) with selected ion mass detection and isotope-dilution techniques. -

1 Abietic Acid R Abrasive Silica for Polishing DR Acenaphthene M (LC
1 abietic acid R abrasive silica for polishing DR acenaphthene M (LC) acenaphthene quinone R acenaphthylene R acetal (see 1,1-diethoxyethane) acetaldehyde M (FC) acetaldehyde-d (CH3CDO) R acetaldehyde dimethyl acetal CH acetaldoxime R acetamide M (LC) acetamidinium chloride R acetamidoacrylic acid 2- NB acetamidobenzaldehyde p- R acetamidobenzenesulfonyl chloride 4- R acetamidodeoxythioglucopyranose triacetate 2- -2- -1- -β-D- 3,4,6- AB acetamidomethylthiazole 2- -4- PB acetanilide M (LC) acetazolamide R acetdimethylamide see dimethylacetamide, N,N- acethydrazide R acetic acid M (solv) acetic anhydride M (FC) acetmethylamide see methylacetamide, N- acetoacetamide R acetoacetanilide R acetoacetic acid, lithium salt R acetobromoglucose -α-D- NB acetohydroxamic acid R acetoin R acetol (hydroxyacetone) R acetonaphthalide (α)R acetone M (solv) acetone ,A.R. M (solv) acetone-d6 RM acetone cyanohydrin R acetonedicarboxylic acid ,dimethyl ester R acetonedicarboxylic acid -1,3- R acetone dimethyl acetal see dimethoxypropane 2,2- acetonitrile M (solv) acetonitrile-d3 RM acetonylacetone see hexanedione 2,5- acetonylbenzylhydroxycoumarin (3-(α- -4- R acetophenone M (LC) acetophenone oxime R acetophenone trimethylsilyl enol ether see phenyltrimethylsilyl... acetoxyacetone (oxopropyl acetate 2-) R acetoxybenzoic acid 4- DS acetoxynaphthoic acid 6- -2- R 2 acetylacetaldehyde dimethylacetal R acetylacetone (pentanedione -2,4-) M (C) acetylbenzonitrile p- R acetylbiphenyl 4- see phenylacetophenone, p- acetyl bromide M (FC) acetylbromothiophene 2- -5- -

Pinacol Rearrangement
Pinacol rearrangement The pinacol–pinacolone rearrangement is a method for converting a 1,2-diol to a carbonyl compound in organic chemistry. The 1,2-rearrangement takes place under acidic conditions. The name of the rearrangement reaction comes from the rearrangement of pinacol to pinacolone.[1] This reaction was first described by Wilhelm Rudolph Fittig in 1860 of the famed Fittig reaction involving coupling of 2 aryl halides in presence of sodium metal in dry ethereal solution.[2] Contents Mechanism Example of asymmetrical pinacol rearrangement Stereochemistry of the rearrangement History See also References Mechanism In the course of this organic reaction, protonation of one of the –OH groups occurs and a carbocation is formed. If the – OH groups are not alike (i.e. the pinacol is asymmetrical), then the one which creates a more stable carbocation participates in the reaction. Subsequently, an alkyl group from the adjacent carbon migrates to the carbocation center. The driving force for this rearrangement step is believed to be the relative stability of the resultant oxonium ion. Although the initial carbocation is already tertiary, the oxygen can stabilize the positive charge much more favorably due to the complete octet configuration at all centers. It can also be seen as the -OH's lone pairs pushing an alkyl group off as seen in the asymmetrical pinacol example. The migration of alkyl groups in this reaction occurs in accordance with their usual migratory aptitude, i.e.hydride > phenyl carbanion > tertiary carbanion (if formed by migration) > secondary carbanion (if formed by migration) > methyl carbanion. {Why carbanion? Because every migratory group leaves by taking electron pair with it.} The conclusion is that the group which stabilizes the carbocation more effectively is migrated.