Reproduction of Earthworms: Sexual Selection and Parthenogenesis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Reproduction in Plants Which But, She Has Never Seen the Seeds We Shall Learn in This Chapter
Reproduction in 12 Plants o produce its kind is a reproduction, new plants are obtained characteristic of all living from seeds. Torganisms. You have already learnt this in Class VI. The production of new individuals from their parents is known as reproduction. But, how do Paheli thought that new plants reproduce? There are different plants always grow from seeds. modes of reproduction in plants which But, she has never seen the seeds we shall learn in this chapter. of sugarcane, potato and rose. She wants to know how these plants 12.1 MODES OF REPRODUCTION reproduce. In Class VI you learnt about different parts of a flowering plant. Try to list the various parts of a plant and write the Asexual reproduction functions of each. Most plants have In asexual reproduction new plants are roots, stems and leaves. These are called obtained without production of seeds. the vegetative parts of a plant. After a certain period of growth, most plants Vegetative propagation bear flowers. You may have seen the It is a type of asexual reproduction in mango trees flowering in spring. It is which new plants are produced from these flowers that give rise to juicy roots, stems, leaves and buds. Since mango fruit we enjoy in summer. We eat reproduction is through the vegetative the fruits and usually discard the seeds. parts of the plant, it is known as Seeds germinate and form new plants. vegetative propagation. So, what is the function of flowers in plants? Flowers perform the function of Activity 12.1 reproduction in plants. Flowers are the Cut a branch of rose or champa with a reproductive parts. -

Reproduction in Fungi: Genetical and Physiological Aspects by CHARLES G
J. Genet., Vol. 73, Number 1, April 1994, pp. 55-56. (~) Printed in India. BOOK REVIEW Reproduction in Fungi: Genetical and Physiological Aspects By CHARLES G. ELLIOTT; Chapman & Hall, London, 1994; 310 pages; s 35.00 It is now ahnost one hundred years since Klebs (1986)laid the foundation of a scientific approach to the study of reproduction in fungi. Since then an extensive literature has accumulated on reproduction in fungi. To present this information ill terms of principles and concepts and to highlight the immediate problems for study is a daunting task. C. G. Elliott has made a commendable attempt in presenting an overview of the genetical and physiological aspects of fungal reproduction. Fungi are remarkable for producing many kinds of reproductive structures in many ways. They produce spores both asexually and sexually. A single fungus produces a single kind of sexual spore: oospores in phycomycetes, ascospores in ascmnycetes and basidiospores in basidiomycetes. The underlying genetical and physiological mechanisms in the formation of spores are complex and we are only beginning to discern them. The introductory chapter examines the advantages of both asexual and sexual reproduction and the persistence of sex in the face of the apparent advantage of asexual reproduction. The reader is reminded that sexual reproduction in fungi not only serves to generate variability, but also provides a means of repairing damaged DNA. Moreover, it can give rise to propagules resistant to unfavourable environmental conditions. In the chapters that follow, one learns that superimposed on the basic event of nuclear fusion are a variety of regulating medhanisms, the effects of which are to determine a successful mating. -

THE Fungus FILES 31 REPRODUCTION & DEVELOPMENT
Reproduction and Development SPORES AND SO MUCH MORE! At any given time, the air we breathe is filled with the spores of many different types of fungi. They form a large proportion of the “flecks” that are seen when direct sunlight shines into a room. They are also remarkably small; 1800 spores could fit lined up on a piece of thread 1 cm long. Fungi typically release extremely high numbers of spores at a time as most of them will not germinate due to landing on unfavourable habitats, being eaten by invertebrates, or simply crowded out by intense competition. A mid-sized gilled mushroom will release up to 20 billion spores over 4-6 days at a rate of 100 million spores per hour. One specimen of the common bracket fungus (Ganoderma applanatum) can produce 350 000 spores per second which means 30 billion spores a day and 4500 billion in one season. Giant puffballs can release a number of spores that number into the trillions. Spores are dispersed via wind, rain, water currents, insects, birds and animals and by people on clothing. Spores contain little or no food so it is essential they land on a viable food source. They can also remain dormant for up to 20 years waiting for an opportune moment to germinate. WHAT ABOUT LIGHT? Though fungi do not need light for food production, fruiting bodies generally grow toward a source of light. Light levels can affect the release of spores; some fungi release spores in the absence of light whereas others (such as the spore throwing Pilobolus) release during the presence of light. -

Rare Parthenogenic Reproduction in a Common Reef Coral, Porites Astreoides Alicia A
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by NSU Works Nova Southeastern University NSUWorks HCNSO Student Theses and Dissertations HCNSO Student Work 1-26-2018 Rare Parthenogenic Reproduction in a Common Reef Coral, Porites astreoides Alicia A. Vollmer [email protected] Follow this and additional works at: https://nsuworks.nova.edu/occ_stuetd Part of the Marine Biology Commons, and the Oceanography and Atmospheric Sciences and Meteorology Commons Share Feedback About This Item NSUWorks Citation Alicia A. Vollmer. 2018. Rare Parthenogenic Reproduction in a Common Reef Coral, Porites astreoides. Master's thesis. Nova Southeastern University. Retrieved from NSUWorks, . (464) https://nsuworks.nova.edu/occ_stuetd/464. This Thesis is brought to you by the HCNSO Student Work at NSUWorks. It has been accepted for inclusion in HCNSO Student Theses and Dissertations by an authorized administrator of NSUWorks. For more information, please contact [email protected]. Thesis of Alicia A. Vollmer Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Science M.S. Marine Biology M.S. Coastal Zone Management Nova Southeastern University Halmos College of Natural Sciences and Oceanography January 2018 Approved: Thesis Committee Major Professor: Nicole Fogarty Committee Member: Joana Figueiredo Committee Member: Xaymara Serrano This thesis is available at NSUWorks: https://nsuworks.nova.edu/occ_stuetd/464 HALMOS COLLEGE OF NATURAL SCIENCES AND OCEANOGRAPHY RARE PARTHENOGENIC REPRODUCTION IN A COMMON REEF CORAL, PORITES ASTREOIDES By Alicia A. Vollmer Submitted to the Faculty of Halmos College of Natural Sciences and Oceanography in partial fulfillment of the requirements for the degree of Master of Science with a specialty in: Marine Biology and Coastal Zone Management Nova Southeastern University January 26, 2018 Thesis of Alicia A. -

Parthenogensis
PARTHENOGENSIS Parthenogenesis is the development of an egg without fertilization. (Gr.Parthenos=virgin; gensis=birth). The individuals formed by parthenogenesis are called parthenotes. Parthenogenesis may be of two types. They are natural parthenogenesis and artificial parthenogenesis. 1. NATURAL PARTHENOGENESIS When parthenogenesis occur spontaneously, it is said to be natural parthenogenesis. Parthenogenesis is a regular natural phenomenon in a few groups of animals. Some animals reproduce exclusively by parthenogenesis. 1 In some other species, parthenogenesis alternates with sexual reproduction. Based on this, natural parthenogenesis is divided into two groups, namely complete parthenogenesis and incomplete parthenogenesis. 1) Complete Parthenogenesis In certain animal parthenogenesis is the only method of reproduction. This type of parthenogenesis is called complete or total or obligatory parthenogenesis. Populations exhibiting total parthenogenesis consist entirely of females. There are no males. E.g. Lacerta (lizard). 1) Incomplete Parthenogenesis In some animals parthenogenesis reproduction and sexual reproduction occur alternately. This is called incomplete or cyclical parthenogenesis. 2 Example a. In gallflies, there is one parthenogenetic reproduction and one sexual reproduction per year (P,S,P,S, (P,S,………). b. In aphids, daphnids and rotifers one sexual reproduction occurs in summer after many parthenogenetic reproductions, (P,P,P,P,P,S,…..P,P,P,P,P,S……..P,). Natural parthenogenesis is further classified into two types. They are haploid parthenogenesis or arrhenotoky and diploid parthenogenesis or thelytoky. A. Haploid Parthenogenesis or Arrhenotoky It is the development of a hyploid egg into a haploid animal. All the haploid individulas are males. Arrhenotoky occur in insects, rotifers and arachnids. 3 i. Haploid Parthenogenesis in insects: In insects haploid parthenogenesis is exhibited by hymenoptera, homoptera, colepters and thysanoptera. -
Cellular Reproduction Vocabulary Date:______Use Page Numbers! Assignment #______Block #____ WORD DEFINITION
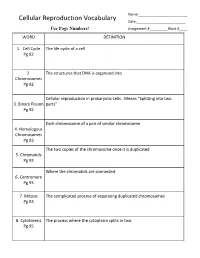
Name:_________________________ Cellular Reproduction Vocabulary Date:_________________________ Use Page Numbers! Assignment #_________ Block #____ WORD DEFINITION 1. Cell Cycle The life cycle of a cell Pg 92 2. The structures that DNA is organized into Chromosomes Pg 92 Cellular reproduction in prokaryotic cells. Means “Splitting into two 3. Binary Fission parts” Pg 92 Each chromosome of a pair of similar chromosome 4. Homologous Chromosomes Pg 93 The two copies of the chromosome once it is duplicated 5. Chromatids Pg 93 Where the chromatids are connected 6. Centromere Pg 93 7. Mitosis The complicated process of separating duplicated chromosomes Pg 93 8. Cytokinesis The process where the cytoplasm splits in two Pg 95 WORD DEFINITION 9. Budding A type of asexual reproduction, where a piece of the parents body Pg 612 develops into an independent organism 10. A type of asexual reproduction, where the organism breaks into two or Regeneration more parts, each growing into a new organism that is genetically identical (fragmentation) to the parent Pg 612 The production of offspring by combining the genetic material of more 11. Sexual than one parent reproduction Pg 613 A single parent has offspring that are identical to itself 12. Asexual reproduction Pg 613 The male sex cell 13. Sperm Pg 613 The female sex cell 14. Egg Pg 613 The new type of cell that is made when an egg’s nucleus fuses with a 15. Zygote sperm’s nucleus Pg 613 16. Spores Small reproductive cells that are protected by a thick cell wall Pg 256 WORD DEFINITION 17. Sex Cells Specialized cells that combine to form a zygote, they have half the normal Pg 114 number of chromosomes, one of each pair. -

Independent Evolution of Sex Chromosomes in Eublepharid Geckos, a Lineage with Environmental and Genotypic Sex Determination
life Article Independent Evolution of Sex Chromosomes in Eublepharid Geckos, A Lineage with Environmental and Genotypic Sex Determination Eleonora Pensabene , Lukáš Kratochvíl and Michail Rovatsos * Department of Ecology, Faculty of Science, Charles University, 12844 Prague, Czech Republic; [email protected] (E.P.); [email protected] (L.K.) * Correspondence: [email protected] or [email protected] Received: 19 November 2020; Accepted: 7 December 2020; Published: 10 December 2020 Abstract: Geckos demonstrate a remarkable variability in sex determination systems, but our limited knowledge prohibits accurate conclusions on the evolution of sex determination in this group. Eyelid geckos (Eublepharidae) are of particular interest, as they encompass species with both environmental and genotypic sex determination. We identified for the first time the X-specific gene content in the Yucatán banded gecko, Coleonyx elegans, possessing X1X1X2X2/X1X2Y multiple sex chromosomes by comparative genome coverage analysis between sexes. The X-specific gene content of Coleonyx elegans was revealed to be partially homologous to genomic regions linked to the chicken autosomes 1, 6 and 11. A qPCR-based test was applied to validate a subset of X-specific genes by comparing the difference in gene copy numbers between sexes, and to explore the homology of sex chromosomes across eleven eublepharid, two phyllodactylid and one sphaerodactylid species. Homologous sex chromosomes are shared between Coleonyx elegans and Coleonyx mitratus, two species diverged approximately 34 million years ago, but not with other tested species. As far as we know, the X-specific gene content of Coleonyx elegans / Coleonyx mitratus was never involved in the sex chromosomes of other gecko lineages, indicating that the sex chromosomes in this clade of eublepharid geckos evolved independently. -

Reproduction in Plants and Animals
Reproduction in Plants and Animals Imagine a gardener checking on his growing plants at the beginning of spring. He notices a few tiny insects eating some of his plants. The gardener isn’t worried—a few insects are not a concern. But when he comes back several weeks later, his plants are covered in these small insects. There are at least ten times as many insects as there were several weeks ago! Where did all of these insects come from? How do organisms make more of their species? Reproduction produces offspring Reproduction is a process by which an organism produces offspring, or young. All organisms reproduce. If they didn’t, no species would survive past a single generation. The tiny insects developing Reproduction allows organisms to pass on their traits, or inside these eggs will grow characteristics to their offspring. Parents pass on their into adult insects. traits through their genetic material, or DNA. Sexual Reproduction requires two parents Sexual reproduction requires a male and female. Each parent contributes half of their genetic material, or DNA, to their offspring. The female contributes her DNA in an egg cell. The male contributes his DNA in a sperm cell. When the egg and sperm combine, they form the new offspring. Offspring may look similar to their parents, but they are not exact copies. In sexual reproduction, each offspring has a mixture of its parent’s traits. Parents may pass on dominant traits or recessive traits to their offspring. Each offspring may be different from its siblings. For These puppies are a product example, suppose the father in a human family does not of sexual reproduction have freckles, but his wife does. -

Living Environment Vocabulary by Prentice Hall 2001 Review Book Unit
Living Environment Vocabulary By Prentice Hall 2001 Review Book Unit Similarities and Topic 1 Differences Among Living Organisms cell the basic unit of structure and function that makes up all organisms metabolism all the chemical reactions that occur within the cells of an organism homeostasis the ability of an organism to maintain a stable internal environment even when the external environment changes reproduction the process by which organisms produce new organisms of the same type cell respiration the process in which nutrients are broken apart, releasing the chemical energy stored in them synthesis a life process that involves combining simple substances into more complex substances organic term used to describe molecules that contain both hydrogen and carbon inorganic a type of molecule that does not contain both carbon and hydrogen but can contain any other combination of elements organelle a structure within the cell that carries out a specific function tissues a group of specialized cells that perform a specific function organ a body structure made of different kinds of tissues combined to perform a specific function organ system several organs that work together to perform a major function in the body cytoplasm the jellylike substance that is between the cell membrane and the nucleus and that contains specialized structures nucleus a large structure within a cell that controls the cell’s metabolism and stores genetic information, including chromosomes and DNA vacuoles storage sacs within the cytoplasm of a cell that may contain -

Asexual Plant Reproduction Diagrams Name: Period
Asexual Plant Reproduction Diagrams Name: Period: Practice: 11 points diagram from The Botany Coloring Book (1982) by Paul Young Asexual Plant Reproduction Diagrams Name: Period: For this assignment, you will be learning some ways that plants can reproduce asexually. Fill in the blanks below using information you learn by coloring the diagram on this assignment. Check off each box ☑ as you finish that part of the instructions. Start by rereading the paragraph titled, “Living Things Reproduce” on p.54, then answer the following questions: 1. The process in which two parents produce offspring that share both parents’ characteristics is called ____________________________________________ . This means that they have a mix of DNA, some from each parent. 2. The process in which one parent produces offspring that are identical to the parent is called ____________________________________________ . This means that they have the same DNA as their parent. 3. Look at the bears in Figure 3. Do they reproduce asexually or sexually? ______________________ 4. Look at the hydra in Figure 4. Do hydras reproduce asexually or sexually? ______________________ Next, go to p. 402-403, read the section titled, “Other Methods of Reproduction”, and then answer the following questions: 5. Besides reproducing sexually, some flowering plants can also reproduce ______________________ . 6. When reproducing asexually, a ______________________ grows from one of the plant parts. 7. ______________________ are above-ground stems from which new plants can grow . 8. ______________________ are tiny new plants that grow along the edges of a plant’s leaves. 9. ______________________ are underground stems that can produce new plants after a dormant season. Look at Figure 5 on p. -

Sex Determination, Sex Ratios and Genetic Conflict
SEX DETERMINATION, SEX RATIOS AND GENETIC CONFLICT John H. Werren1 and Leo W. Beukeboom2 Biology Department, University of Rochester, Rochester, N.Y. 14627 2Institute of Evolutionary and Ecological Sciences, University of Leiden, NL-2300 RA Leiden, The Netherlands 1998. Ann. Rev. Ecol. & Systematics 29:233-261. ABSTRACT Genetic mechanisms of sex determination are unexpectedly diverse and change rapidly during evolution. We review the role of genetic conflict as the driving force behind this diversity and turnover. Genetic conflict occurs when different components of a genetic system are subject to selection in opposite directions. Conflict may occur between genomes (including paternal- maternal and parental-zygotic conflicts), or within genomes (between cytoplasmic and nuclear genes, or sex chromosomes and autosomes). The sex determining system consists of parental sex ratio genes, parental effect sex determiners and zygotic sex determiners, which are subject to different selection pressures due to differences in their modes of inheritance and expression. Genetic conflict theory is used to explain the evolution of several sex determining mechanisms including sex chromosome drive, cytoplasmic sex ratio distorters and cytoplasmic male sterility in plants. Although the evidence is still limited, the role of genetic conflict in sex determination evolution is gaining support. PERSPECTIVES AND OVERVIEW Sex determining mechanisms are incredibly diverse in plants and animals. A brief summary of the diversity will illustrate the point. In hermaphroditic species both male (microgamete) and female (macrogamete) function reside within the same individual, whereas dioecious (or gonochoristic) species have separate sexes. Within these broad categories there is considerable diversity in the phenotypic and genetic mechanisms of sex determination. -

The Evolutionary Significance of Parthenogenesis and Sexual Reproduction In
The evolutionary significance of parthenogenesis and sexual reproduction in the Australian spiny leaf insect, Extatosoma tiaratum Yasaman Alavi Submitted in total fulfilment of the requirements of the degree of Doctor of Philosophy October 2016 School of BioSciences Faculty of Science The University of Melbourne i ii Abstract The costs and benefits of sexual reproduction has long been a subject of debate in biology. The paradox arises from the fact that theoretically, sex is associated with many costs, yet it is the most prevalent mode of reproduction in the tree of life. Facultative parthenogenetic systems, in which females can reproduce both sexually, and in the absence of sperm, parthenogenetically, provide suitable systems to compare costs and benefits of reproductive modes, while minimizing confounding effect that are not directly related to reproductive modes. In this thesis, I used the Australian Phasmatid, Extatosoma tiaratum, to investigate the evolutionary significance of facultative parthenogenesis, and compare fitness consequences of sex and parthenogenesis. The evolutionary significance of facultative parthenogenesis is unknown but male or sperm limitations are potential factors. I investigate male mating frequency and variation in ejaculate size and quality in E. tiaratum. I show that most, but not all, males are able to mate multiply, but ejaculate size decreases with increased number of matings. In addition, ejaculate size increased with increasing time interval between matings, suggesting that E. tiaratum males require time to replenish ejaculate reserves. These findings suggest male sperm limitation may be an important factor influencing the evolution of parthenogenesis in this system. iii The cytological mechanism of parthenogenesis determines the genetic diversity and heterozygosity levels of the offspring and is thus an important component of the comparison between reproductive modes.