THE Fungus FILES 31 REPRODUCTION & DEVELOPMENT
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Reproduction in Plants Which But, She Has Never Seen the Seeds We Shall Learn in This Chapter
Reproduction in 12 Plants o produce its kind is a reproduction, new plants are obtained characteristic of all living from seeds. Torganisms. You have already learnt this in Class VI. The production of new individuals from their parents is known as reproduction. But, how do Paheli thought that new plants reproduce? There are different plants always grow from seeds. modes of reproduction in plants which But, she has never seen the seeds we shall learn in this chapter. of sugarcane, potato and rose. She wants to know how these plants 12.1 MODES OF REPRODUCTION reproduce. In Class VI you learnt about different parts of a flowering plant. Try to list the various parts of a plant and write the Asexual reproduction functions of each. Most plants have In asexual reproduction new plants are roots, stems and leaves. These are called obtained without production of seeds. the vegetative parts of a plant. After a certain period of growth, most plants Vegetative propagation bear flowers. You may have seen the It is a type of asexual reproduction in mango trees flowering in spring. It is which new plants are produced from these flowers that give rise to juicy roots, stems, leaves and buds. Since mango fruit we enjoy in summer. We eat reproduction is through the vegetative the fruits and usually discard the seeds. parts of the plant, it is known as Seeds germinate and form new plants. vegetative propagation. So, what is the function of flowers in plants? Flowers perform the function of Activity 12.1 reproduction in plants. Flowers are the Cut a branch of rose or champa with a reproductive parts. -

Feeding-Dependent Tentacle Development in the Sea Anemone Nematostella Vectensis
bioRxiv preprint doi: https://doi.org/10.1101/2020.03.12.985168; this version posted March 12, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Feeding-dependent tentacle development in the sea anemone Nematostella vectensis Aissam Ikmi1,2*, Petrus J. Steenbergen1, Marie Anzo1, Mason R. McMullen2,3, Anniek Stokkermans1, Lacey R. Ellington2, and Matthew C. Gibson2,4 Affiliations: 1Developmental Biology Unit, European Molecular Biology Laboratory, 69117 Heidelberg, Germany. 2Stowers Institute for Medical Research, Kansas City, Missouri 64110, USA. 3Department of Pharmacy, The University of Kansas Health System, Kansas City, Kansas 66160, USA. 4Department of Anatomy and Cell Biology, The University of Kansas School of Medicine, Kansas City, Kansas 66160, USA. *Corresponding author. Email: [email protected] 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.03.12.985168; this version posted March 12, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Summary In cnidarians, axial patterning is not restricted to embryonic development but continues throughout a prolonged life history filled with unpredictable environmental changes. How this developmental capacity copes with fluctuations of food availability and whether it recapitulates embryonic mechanisms remain poorly understood. To address these questions, we utilize the tentacles of the sea anemone Nematostella vectensis as a novel paradigm for developmental patterning across distinct life history stages. -

Characterization of Two Undescribed Mucoralean Species with Specific
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 26 March 2018 doi:10.20944/preprints201803.0204.v1 1 Article 2 Characterization of Two Undescribed Mucoralean 3 Species with Specific Habitats in Korea 4 Seo Hee Lee, Thuong T. T. Nguyen and Hyang Burm Lee* 5 Division of Food Technology, Biotechnology and Agrochemistry, College of Agriculture and Life Sciences, 6 Chonnam National University, Gwangju 61186, Korea; [email protected] (S.H.L.); 7 [email protected] (T.T.T.N.) 8 * Correspondence: [email protected]; Tel.: +82-(0)62-530-2136 9 10 Abstract: The order Mucorales, the largest in number of species within the Mucoromycotina, 11 comprises typically fast-growing saprotrophic fungi. During a study of the fungal diversity of 12 undiscovered taxa in Korea, two mucoralean strains, CNUFC-GWD3-9 and CNUFC-EGF1-4, were 13 isolated from specific habitats including freshwater and fecal samples, respectively, in Korea. The 14 strains were analyzed both for morphology and phylogeny based on the internal transcribed 15 spacer (ITS) and large subunit (LSU) of 28S ribosomal DNA regions. On the basis of their 16 morphological characteristics and sequence analyses, isolates CNUFC-GWD3-9 and CNUFC- 17 EGF1-4 were confirmed to be Gilbertella persicaria and Pilobolus crystallinus, respectively.To the 18 best of our knowledge, there are no published literature records of these two genera in Korea. 19 Keywords: Gilbertella persicaria; Pilobolus crystallinus; mucoralean fungi; phylogeny; morphology; 20 undiscovered taxa 21 22 1. Introduction 23 Previously, taxa of the former phylum Zygomycota were distributed among the phylum 24 Glomeromycota and four subphyla incertae sedis, including Mucoromycotina, Kickxellomycotina, 25 Zoopagomycotina, and Entomophthoromycotina [1]. -

Reproduction in Fungi: Genetical and Physiological Aspects by CHARLES G
J. Genet., Vol. 73, Number 1, April 1994, pp. 55-56. (~) Printed in India. BOOK REVIEW Reproduction in Fungi: Genetical and Physiological Aspects By CHARLES G. ELLIOTT; Chapman & Hall, London, 1994; 310 pages; s 35.00 It is now ahnost one hundred years since Klebs (1986)laid the foundation of a scientific approach to the study of reproduction in fungi. Since then an extensive literature has accumulated on reproduction in fungi. To present this information ill terms of principles and concepts and to highlight the immediate problems for study is a daunting task. C. G. Elliott has made a commendable attempt in presenting an overview of the genetical and physiological aspects of fungal reproduction. Fungi are remarkable for producing many kinds of reproductive structures in many ways. They produce spores both asexually and sexually. A single fungus produces a single kind of sexual spore: oospores in phycomycetes, ascospores in ascmnycetes and basidiospores in basidiomycetes. The underlying genetical and physiological mechanisms in the formation of spores are complex and we are only beginning to discern them. The introductory chapter examines the advantages of both asexual and sexual reproduction and the persistence of sex in the face of the apparent advantage of asexual reproduction. The reader is reminded that sexual reproduction in fungi not only serves to generate variability, but also provides a means of repairing damaged DNA. Moreover, it can give rise to propagules resistant to unfavourable environmental conditions. In the chapters that follow, one learns that superimposed on the basic event of nuclear fusion are a variety of regulating medhanisms, the effects of which are to determine a successful mating. -

<I>Mucorales</I>
Persoonia 30, 2013: 57–76 www.ingentaconnect.com/content/nhn/pimj RESEARCH ARTICLE http://dx.doi.org/10.3767/003158513X666259 The family structure of the Mucorales: a synoptic revision based on comprehensive multigene-genealogies K. Hoffmann1,2, J. Pawłowska3, G. Walther1,2,4, M. Wrzosek3, G.S. de Hoog4, G.L. Benny5*, P.M. Kirk6*, K. Voigt1,2* Key words Abstract The Mucorales (Mucoromycotina) are one of the most ancient groups of fungi comprising ubiquitous, mostly saprotrophic organisms. The first comprehensive molecular studies 11 yr ago revealed the traditional Mucorales classification scheme, mainly based on morphology, as highly artificial. Since then only single clades have been families investigated in detail but a robust classification of the higher levels based on DNA data has not been published phylogeny yet. Therefore we provide a classification based on a phylogenetic analysis of four molecular markers including the large and the small subunit of the ribosomal DNA, the partial actin gene and the partial gene for the translation elongation factor 1-alpha. The dataset comprises 201 isolates in 103 species and represents about one half of the currently accepted species in this order. Previous family concepts are reviewed and the family structure inferred from the multilocus phylogeny is introduced and discussed. Main differences between the current classification and preceding concepts affects the existing families Lichtheimiaceae and Cunninghamellaceae, as well as the genera Backusella and Lentamyces which recently obtained the status of families along with the Rhizopodaceae comprising Rhizopus, Sporodiniella and Syzygites. Compensatory base change analyses in the Lichtheimiaceae confirmed the lower level classification of Lichtheimia and Rhizomucor while genera such as Circinella or Syncephalastrum completely lacked compensatory base changes. -

Feeding-Dependent Tentacle Development in the Sea Anemone Nematostella Vectensis ✉ Aissam Ikmi 1,2 , Petrus J
ARTICLE https://doi.org/10.1038/s41467-020-18133-0 OPEN Feeding-dependent tentacle development in the sea anemone Nematostella vectensis ✉ Aissam Ikmi 1,2 , Petrus J. Steenbergen1, Marie Anzo 1, Mason R. McMullen2,3, Anniek Stokkermans1, Lacey R. Ellington2 & Matthew C. Gibson2,4 In cnidarians, axial patterning is not restricted to embryogenesis but continues throughout a prolonged life history filled with unpredictable environmental changes. How this develop- 1234567890():,; mental capacity copes with fluctuations of food availability and whether it recapitulates embryonic mechanisms remain poorly understood. Here we utilize the tentacles of the sea anemone Nematostella vectensis as an experimental paradigm for developmental patterning across distinct life history stages. By analyzing over 1000 growing polyps, we find that tentacle progression is stereotyped and occurs in a feeding-dependent manner. Using a combination of genetic, cellular and molecular approaches, we demonstrate that the crosstalk between Target of Rapamycin (TOR) and Fibroblast growth factor receptor b (Fgfrb) signaling in ring muscles defines tentacle primordia in fed polyps. Interestingly, Fgfrb-dependent polarized growth is observed in polyp but not embryonic tentacle primordia. These findings show an unexpected plasticity of tentacle development, and link post-embryonic body patterning with food availability. 1 Developmental Biology Unit, European Molecular Biology Laboratory, 69117 Heidelberg, Germany. 2 Stowers Institute for Medical Research, Kansas City, MO 64110, -

PLANTS MUST DISPERSE THEIR SEEDS 8I
8o YEARBOOK OF AGRICULTURE 1961 may resist germination for long periods. Some species produce pods in which one segment remains indéhiscent— closed—and the seed within it remains Plants Must Disperse dormant for a long time, as in cockle- bur (Xanthium), for example. Their Seeds THE DISPERSAL of seeds is determined largely by the size, shape, and char- acter of the seedcoat or the persisting PAUL G. RUSSELL AND ALBINA F. MUSIL structures of the fruit as, for example, the awns of grasses; the ''fuzz" of cot- ton; spines and bristles of various NOT ALL SEEDS survive the struggle for forms; "wings" on the seeds of certain existence. Any marked change in trees; plumes of dandelion and thistle; environment, moisture, temperature, the forceful opening of the seed pod, amount of sunlight, or soil composition as in witch-hazel {Hamamelis virginica) ; may create conditions under which and a sticky surface when wet. seeds of certain plants cannot germi- Such seeds are dispersed readily by nate. Plants therefore must disperse such, natural means as wind, water, their seeds in such a manner and animals, and birds. in such quantity that some, at least, When structures, such as awns and will survive so that the species may pubescence, have been removed in the continue. process of harvesting and cleaning of Devices for survival among plants crop seeds, such seeds may become are many. widely distributed in any of several The dormant embryonic plant with- ways—with crop seeds, feeds (hay and in the seed of most kinds of plants is grain), common carriers (trucks, auto- protected by a seedcoat until condi- mobiles, wagons, airplanes), farm im- tions are favorable for new grow^th to plements, ships, birds, and insects. -

Tumbleweed [Poems
Class ^S3& 'M Boo]c.__J2L^JlIi Copyright ]J^ \^0'h COPYRIGHT DEPOSm TUMBLBWBBD. Copyright, 1909, by H, Rea Woodman LiBRARY of CONGRESS «<> '-"Dies Received Printed in March. 190si f APR 27 1909 Copyri^nt Entry _ CLASS 0L_ '^'^'^ '*'*'' As I used to bring my verses to you when I was a little girl, to whom else should I turn now, dear father? With the same joyous love, and confidence in your sympathy, I bring you these vagrants from the prairies you loved with all the passion of your poet-mind. In reverence and gratitude and longing I write upon this page the supremest name to me, the name of the richest-hearted man I have known, my father, William Clayton Woodman. CONTENTS The Tumbleweed 1 Lakeside 3 "Green's Gittin' On in the World" 4 A Birthday 6 The Palms 7 April Nineteenth 9 "Oh, That's All Off!" 10 A Forgiveness 11 "Unto All Their Due" 13 The Three Lights . 14 When We Swear Teddy In 15 At My Window 16 To Omar 17 The First Easter 18 Two Wrncs 19 I Am Free ! I Am Free ! 21 An Ultra-Rational View 23 "Not a Sparrow Falleth" 24 My Poet 25 An Appreciation 26 A Minor Tragedy 28 The Sure Road 29 Two Worlds 31 Real Enjoyment 32 Perhaps, Tom 34 Minnie Adelaide Angeline 35 The Unknown 36 All Day 37 If My Halting Pen Leaves One Trace .... 38 "Them Browns" 39 summum bon^um 41 The Leisuee Sex 43 Broken 44 As the Crimsox Dies 45 Two Aprils 47 The Caged Sikgers 48 Responsibh-ity 49 Concerning Us, Dear 50 "OLE Ashus!" 51 What Do I Thank You for Most, Oh Ideal? . -

Common Edible Plants of Africa
Domesticates Geographical Distribution Morphology/Description Common, edible fruits Oil Palm Tropical Africa, cannot tolerate full A tree. The oil palm is now one of the most economically Elaeis guineensis shade, but prefers disturbed important palms in Africa. It has a walnut-size fruit habitats5 clustered in big pods, with a fibrous pulp rich in oil (which is rich in energy, fatty acids, and a great source of Vitamin West African origins, but has 6, A). Within the husk is a hard-shelled seed containing an spread throughout tropical Africa edible kernel (eaten by chimps and people). (The sap is tapped to make palm wine too.) The species still grows wild, as well as being cultivated and planted by people. The wild form growing in the Ituri Forest in the Congo, provides 9% of the total caloric intake for the Efe pygimies, for example (Bailey and Peacock 1988, McGrew 1992). Okra Savanna, full sun areas Possible originated in East Africa6 Hibiscus esculentus5 Melon Continent Wild varieties of this melon still grow in many arid and Citrullus lanatus5 semi-arid regions of the continent. They are smaller, and more bitter/toxic than the domestic versions. Gourd Tropical Africa Lagenaria siceraria7 Desert Date Dry regions of the continent Scrambling shrub. Fruits are 1-2 inches long, with fibrous, Balanites aegyptiaca oily flesh and large seed. Baobab Widespread in south-central Africa Large tree with huge trunk. Dry, fleshy pods 8-10 inches Adansonia digitata in semi arid regions long containing numerous seeds P380: Common edible plants of Africa - 1 - Horned melon, wild cucumber Widespread in Savannas Wild varieties of cucumis, the cucumber genus, grow Cucumis (many species) widely as spreading vines on the ground in savanna regions. -

Rare Parthenogenic Reproduction in a Common Reef Coral, Porites Astreoides Alicia A
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by NSU Works Nova Southeastern University NSUWorks HCNSO Student Theses and Dissertations HCNSO Student Work 1-26-2018 Rare Parthenogenic Reproduction in a Common Reef Coral, Porites astreoides Alicia A. Vollmer [email protected] Follow this and additional works at: https://nsuworks.nova.edu/occ_stuetd Part of the Marine Biology Commons, and the Oceanography and Atmospheric Sciences and Meteorology Commons Share Feedback About This Item NSUWorks Citation Alicia A. Vollmer. 2018. Rare Parthenogenic Reproduction in a Common Reef Coral, Porites astreoides. Master's thesis. Nova Southeastern University. Retrieved from NSUWorks, . (464) https://nsuworks.nova.edu/occ_stuetd/464. This Thesis is brought to you by the HCNSO Student Work at NSUWorks. It has been accepted for inclusion in HCNSO Student Theses and Dissertations by an authorized administrator of NSUWorks. For more information, please contact [email protected]. Thesis of Alicia A. Vollmer Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Science M.S. Marine Biology M.S. Coastal Zone Management Nova Southeastern University Halmos College of Natural Sciences and Oceanography January 2018 Approved: Thesis Committee Major Professor: Nicole Fogarty Committee Member: Joana Figueiredo Committee Member: Xaymara Serrano This thesis is available at NSUWorks: https://nsuworks.nova.edu/occ_stuetd/464 HALMOS COLLEGE OF NATURAL SCIENCES AND OCEANOGRAPHY RARE PARTHENOGENIC REPRODUCTION IN A COMMON REEF CORAL, PORITES ASTREOIDES By Alicia A. Vollmer Submitted to the Faculty of Halmos College of Natural Sciences and Oceanography in partial fulfillment of the requirements for the degree of Master of Science with a specialty in: Marine Biology and Coastal Zone Management Nova Southeastern University January 26, 2018 Thesis of Alicia A. -
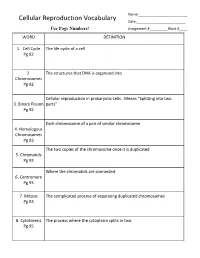
Cellular Reproduction Vocabulary Date:______Use Page Numbers! Assignment #______Block #____ WORD DEFINITION
Name:_________________________ Cellular Reproduction Vocabulary Date:_________________________ Use Page Numbers! Assignment #_________ Block #____ WORD DEFINITION 1. Cell Cycle The life cycle of a cell Pg 92 2. The structures that DNA is organized into Chromosomes Pg 92 Cellular reproduction in prokaryotic cells. Means “Splitting into two 3. Binary Fission parts” Pg 92 Each chromosome of a pair of similar chromosome 4. Homologous Chromosomes Pg 93 The two copies of the chromosome once it is duplicated 5. Chromatids Pg 93 Where the chromatids are connected 6. Centromere Pg 93 7. Mitosis The complicated process of separating duplicated chromosomes Pg 93 8. Cytokinesis The process where the cytoplasm splits in two Pg 95 WORD DEFINITION 9. Budding A type of asexual reproduction, where a piece of the parents body Pg 612 develops into an independent organism 10. A type of asexual reproduction, where the organism breaks into two or Regeneration more parts, each growing into a new organism that is genetically identical (fragmentation) to the parent Pg 612 The production of offspring by combining the genetic material of more 11. Sexual than one parent reproduction Pg 613 A single parent has offspring that are identical to itself 12. Asexual reproduction Pg 613 The male sex cell 13. Sperm Pg 613 The female sex cell 14. Egg Pg 613 The new type of cell that is made when an egg’s nucleus fuses with a 15. Zygote sperm’s nucleus Pg 613 16. Spores Small reproductive cells that are protected by a thick cell wall Pg 256 WORD DEFINITION 17. Sex Cells Specialized cells that combine to form a zygote, they have half the normal Pg 114 number of chromosomes, one of each pair. -

Morphological and Molecular Characterization of Ascobolus and Pilobolus Fungi in Wild Herbivore Dung in Nairobi National Park Mi
MORPHOLOGICAL AND MOLECULAR CHARACTERIZATION OF ASCOBOLUS AND PILOBOLUS FUNGI IN WILD HERBIVORE DUNG IN NAIROBI NATIONAL PARK MIYUNGA ANTOINETTE ALUOCH A Research Thesis Submitted to the Graduate School in Partial Fulfilment for the Requirements of the Award of Master of Science Degree in Biochemistry of Egerton University EGERTON UNIVERSITY NOVEMBER, 2015 DECLARATION AND RECOMMENDATION Declaration This thesis is my original work and has not been submitted or presented for examination in any institution Miyunga Antoinette Aluoch SM14/3263/12 Signature..................................................... Date………………………………….. Recommendation This thesis has been submitted for examination with our approval as supervisors Dr. Meshack Obonyo Senior Lecturer Department of Biochemistry and Molecular Biology Egerton University Signature..................................................... Date………………………………….. Dr. Daniel Okun Lecturer Department of Biochemistry and Biotechnology Kenyatta University Signature..................................................... Date………………………………….. ii COPYRIGHT ©2015 Miyunga A Aluoch No parts of this work may be reproduced, stored in a retrieval system or transmitted by any means, mechanical photocopying and electronic process, recording or otherwise copied for public or private use without the prior written permission from Egerton University. All rights reserved. iii DEDICATION This thesis is dedicated to my family for their love and support always. iv ACKNOWLEDGEMENT I thank my supervisors Dr. Meshack Obonyo of Department