Structure and Distribution of Chalky Deposits in the Pacific Oyster Using
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Common Name: Chiton Class: Polyplacophora
Common Name: Chiton Class: Polyplacophora Scrapes algae off rock with radula 8 Overlapping Plates Phylum? Mollusca Class? Gastropoda Common name? Brown sea hare Class? Scaphopoda Common name? Tooth shell or tusk shell Mud Tentacle Foot Class? Gastropoda Common name? Limpet Phylum? Mollusca Class? Bivalvia Class? Gastropoda Common name? Brown sea hare Phylum? Mollusca Class? Gastropoda Common name? Nudibranch Class? Cephalopoda Cuttlefish Octopus Squid Nautilus Phylum? Mollusca Class? Gastropoda Most Bivalves are Filter Feeders A B E D C • A: Mantle • B: Gill • C: Mantle • D: Foot • E: Posterior adductor muscle I.D. Green: Foot I.D. Red Gills Three Body Regions 1. Head – Foot 2. Visceral Mass 3. Mantle A B C D • A: Radula • B: Mantle • C: Mouth • D: Foot What are these? Snail Radulas Dorsal HingeA Growth line UmboB (Anterior) Ventral ByssalC threads Mussel – View of Outer Shell • A: Hinge • B: Umbo • C: Byssal threads Internal Anatomy of the Bay Mussel A B C D • A: Labial palps • B: Mantle • C: Foot • D: Byssal threads NacreousB layer Posterior adductorC PeriostracumA muscle SiphonD Mantle Byssal threads E Internal Anatomy of the Bay Mussel • A: Periostracum • B: Nacreous layer • C: Posterior adductor muscle • D: Siphon • E: Mantle Byssal gland Mantle Gill Foot Labial palp Mantle Byssal threads Gill Byssal gland Mantle Foot Incurrent siphon Byssal Labial palp threads C D B A E • A: Foot • B: Gills • C: Posterior adductor muscle • D: Excurrent siphon • E: Incurrent siphon Heart G F H E D A B C • A: Foot • B: Gills • C: Mantle • D: Excurrent siphon • E: Incurrent siphon • F: Posterior adductor muscle • G: Labial palps • H: Anterior adductor muscle Siphon or 1. -

Silicified Eocene Molluscs from the Lower Murchison District, Southern Carnarvon Basin, Western Australia
[<ecords o{ the Western A uslralian Museum 24: 217--246 (2008). Silicified Eocene molluscs from the Lower Murchison district, Southern Carnarvon Basin, Western Australia Thomas A. Darragh1 and George W. Kendrick2.3 I Department of Invertebrate Palaeontology, Museum Victoria, 1'.0. Box 666, Melbourne, Victoria 3001, Australia. Email: tdarragh(il.Illuseum.vic.gov.au :' Department of Earth and Planetary Sciences, Western Australian Museum, Locked Bag 49, Welshpool D.C., Western Australia 6986, Australia. 1 School of Earth and Ceographical Sciences, The University of Western Australia, 35 Stirling Highway, Crawlev, Western Australia 6009, Australia. Abstract - Silicified Middle to Late Eocene shallow water sandstones outcropping in the Lower Murchison District near Kalbarri township contain a silicified fossil fauna including foraminifera, sponges, bryozoans, solitary corals, brachiopods, echinoids and molluscs. The known molluscan fauna consists of 51 species, comprising 2 cephalopods, 14 bivalves, 1 scaphopod and 34 gastropods. Of these taxa three are newly described, Cerithium lvilya, Zeacolpus bartol1i, and Lyria lamellatoplicata. 25 of these molluscs are identical to or closely comparable with taxa from the southern Australian Eocene. The occurrence of this fauna extends the Southeast Australian Province during the Eocene from southwest Western Australia along the west coast north to at least 27° present day south latitude; consequently the province is here renamed the Southern Australian Province. Keywords: siliceous fossils, Eocene, Kalbarri, molluscs, new taxa, Carnarvon Basin, biogeography, Southern Australian Province. INTRODUCTION The source deposit, a pallid to ferruginous silicified Eocene marine molluscan assemblages from sandstone, forms a weakly defined, low breakaway coastal sedimentary basins in southern Australia trending N-S and sloping gently westward. -
Field Identification Guide to the Living Marine Resources In
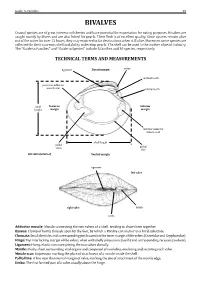
Guide to Families 29 BIVALVES Coastal species are of great interest to fisheries and have potential for exportation for eating purposes. Bivalves are caught mainly by divers and are also fished for pearls. Their flesh is of excellent quality. Since oysters remain alive out of the water for over 12 hours, they may exported to far destinations when still alive. Moreover, some species are collected for their nacreous shell and ability to develop pearls. The shell can be used in the mother of pearl industry. The “Guide to Families’’ andTECHNICAL ‘‘Guide to Species’’ TERMS include 5AND families MEASUREMENTS and 10 species, respectively. ligament Dorsal margin umbo posterior adductor cardinal tooth muscle scar lateral tooth Posterior Anterior margin margin shell height anterior adductor muscle scar pallial sinus pallial shell length line left valve (interior) Ventral margin ligament left valve right valve lunule umbo Adductor muscle: Byssus: Chomata: Muscle connecting the two valves of a shell, tending to draw them together. Hinge: Clump of horny threads spun by the foot, by which a Bivalve can anchor to a hard substrate. Ligament: Small denticles and corresponding pits located on the inner margin of the valves (Ostreidae and Gryphaeidae). Mantle: Top interlocking margin of the valves, often with shelly projections (teeth) and corresponding recesses (sockets). Muscle scar: Horny, elastic structure joining the two valves dorsally. Pallial line: Fleshy sheet surrounding vital organs and composed of two lobes, one lining and secreting each valve. Umbo: Impression marking the place of attachment of a muscle inside the shell. A line near the internal margin of valve, marking the site of attachment of the mantle edge. -

Biochemical Composition and Condition of Crassostrea Gigas (Thunberg, 1793) in Relation to Integrated Multi-Trophic Aquaculture (IMTA) Feed Sources
Biochemical composition and condition of Crassostrea gigas (Thunberg, 1793) in relation to integrated multi-trophic aquaculture (IMTA) feed sources vorgelegt von: Maximilian Felix Schupp geb. am 06.06.1990 in Lippstadt Erstgutachter: Zweitgutachter: Dr. Adrian Bischoff-Lang Prof. Dr. Bela H. Buck Universität Rostock Alfred-Wegener-Institut Agrar- und Umweltwissenschaftliche Helmholtz Zentrum für Polar Fakultät und Meeresforschung (AWI) Lehrstuhl Aquakultur und Sea-Ranching MASTERARBEIT im Studiengang Aquakultur und Sea-Ranching Agrar- und Umweltwissenschaftliche Fakultät Rostock, 2015 Directory 1 INTRODUCTION .................................................................................................. 1 2 BACKGROUND ..................................................................................................... 5 2.1 Biology of Crassostrea gigas ............................................................................................................. 5 2.2 Lipids and fatty acids...................................................................................................................... 10 3 MATERIALS AND METHODS ......................................................................... 12 3.1 Specimen .......................................................................................................................................... 12 3.2 Experimental setup ......................................................................................................................... 12 3.3 Determination of dry weight -

Clam Dissection Guideline
Clam Dissection Guideline BACKGROUND: Clams are bivalves, meaning that they have shells consisting of two halves, or valves. The valves are joined at the top, and the adductor muscles on each side hold the shell closed. If the adductor muscles are relaxed, the shell is pulled open by ligaments located on each side of the umbo. The clam's foot is used to dig down into the sand, and a pair of long incurrent and excurrent siphons that extrude from the clam's mantle out the side of the shell reach up to the water above (only the exit points for the siphons are shown). Clams are filter feeders. Water and food particles are drawn in through one siphon to the gills where tiny, hair-like cilia move the water, and the food is caught in mucus on the gills. From there, the food-mucus mixture is transported along a groove to the palps (mouth flaps) which push it into the clam's mouth. The second siphon carries away the water. The gills also draw oxygen from the water flow. The mantle, a thin membrane surrounding the body of the clam, secretes the shell. The oldest part of the clam shell is the umbo, and it is from the hinge area that the clam extends as it grows. I. Purpose: The purpose of this lab is to identify the internal and external structures of a mollusk by dissecting a clam. II. Materials: 2 pairs of safety goggles 1 paper towel 2 pairs of gloves 1 pair of scissors 1 preserved clam 2 pairs of forceps 1 dissecting tray 2 probes III. -

Annual Report Fy2016
ANNUAL REPORT FY2016 AFFILIATED WITH Affiliated with Cornell University PRI: WHO WE ARE Founded in 1932, the Paleontological Research Institution (PRI) pursues and integrates education and research, and interprets the history and systems of the Earth and its life. Our aim is to increase knowledge, educate society, and encourage wise stewardship of the Earth. PRI has two campuses and one large plot of forest property north of Ithaca, NY. Palmer Hall Museum of the Earth Named in honor of Katherine Palmer Opened in 2003, the Museum of the Earth (Director, 1952-1978), Palmer Hall is the is home to temporary and permanent Institution’s main building, housing PRI’s exhibitions that teach visitors about the collections, laboratories, library, and offices. history of life on Earth. Cayuga Nature Center Smith Woods The Cayuga Nature Center merged with Located in Trumansburg, NY, Smith Woods PRI in 2013. The Nature Center’s education is the largest plot of old-growth forest in programs and exhibitions focus on the central New York. More than 32 acres large, natural history of the Cayuga Lake basin, Smith Woods serves as a research and and are conducted in the Lodge and on the education resource for elementary through 120 acres of woodlands and fields on-site. graduate students. TABLE OF CONTENTS DIRECTOR’S AND PRESIDENT’S MESSAGE 2-3 PRI SERVES: 2016-2016 AT A GLANCE 4-5 RESEARCH 6-9 PUBLICATIONS 10-11 COLLECTIONS 12-13 EDUCATION 14-18 GRANTS 19 CORNELL UNIVERSITY RELATIONS 20-23 MUSEUM OF THE EARTH 24-25 CAYUGA NATURE CENTER 26-27 EXHIBITIONS 28-31 COMMUNITY ACCESSIBILITY 32-33 INTERNS AND VOLUNTEERS 34-35 DONOR SUPPORT 36-39 FINANCIAL ACTIVITY STATEMENT 40 BOARD OF TRUSTEES AND STAFF 41 FRONT COVER BACKGROUND IMAGE: Blue sky at the Cayuga Nature Center. -

Early Ontogeny of Jurassic Bakevelliids and Their Bearing on Bivalve Evolution
Early ontogeny of Jurassic bakevelliids and their bearing on bivalve evolution NIKOLAUS MALCHUS Malchus, N. 2004. Early ontogeny of Jurassic bakevelliids and their bearing on bivalve evolution. Acta Palaeontologica Polonica 49 (1): 85–110. Larval and earliest postlarval shells of Jurassic Bakevelliidae are described for the first time and some complementary data are given concerning larval shells of oysters and pinnids. Two new larval shell characters, a posterodorsal outlet and shell septum are described. The outlet is homologous to the posterodorsal notch of oysters and posterodorsal ridge of arcoids. It probably reflects the presence of the soft anatomical character post−anal tuft, which, among Pteriomorphia, was only known from oysters. A shell septum was so far only known from Cassianellidae, Lithiotidae, and the bakevelliid Kobayashites. A review of early ontogenetic shell characters strongly suggests a basal dichotomy within the Pterio− morphia separating taxa with opisthogyrate larval shells, such as most (or all?) Praecardioida, Pinnoida, Pterioida (Bakevelliidae, Cassianellidae, all living Pterioidea), and Ostreoida from all other groups. The Pinnidae appear to be closely related to the Pterioida, and the Bakevelliidae belong to the stem line of the Cassianellidae, Lithiotidae, Pterioidea, and Ostreoidea. The latter two superfamilies comprise a well constrained clade. These interpretations are con− sistent with recent phylogenetic hypotheses based on palaeontological and genetic (18S and 28S mtDNA) data. A more detailed phylogeny is hampered by the fact that many larval shell characters are rather ancient plesiomorphies. Key words: Bivalvia, Pteriomorphia, Bakevelliidae, larval shell, ontogeny, phylogeny. Nikolaus Malchus [[email protected]], Departamento de Geologia/Unitat Paleontologia, Universitat Autòno− ma Barcelona, 08193 Bellaterra (Cerdanyola del Vallès), Spain. -

Olympia Oyster (Ostrea Lurida)
COSEWIC Assessment and Status Report on the Olympia Oyster Ostrea lurida in Canada SPECIAL CONCERN 2011 COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows: COSEWIC. 2011. COSEWIC assessment and status report on the Olympia Oyster Ostrea lurida in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xi + 56 pp. (www.sararegistry.gc.ca/status/status_e.cfm). Previous report(s): COSEWIC. 2000. COSEWIC assessment and status report on the Olympia Oyster Ostrea conchaphila in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vii + 30 pp. (www.sararegistry.gc.ca/status/status_e.cfm) Gillespie, G.E. 2000. COSEWIC status report on the Olympia Oyster Ostrea conchaphila in Canada in COSEWIC assessment and update status report on the Olympia Oyster Ostrea conchaphila in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 1-30 pp. Production note: COSEWIC acknowledges Graham E. Gillespie for writing the provisional status report on the Olympia Oyster, Ostrea lurida, prepared under contract with Environment Canada and Fisheries and Oceans Canada. The contractor’s involvement with the writing of the status report ended with the acceptance of the provisional report. Any modifications to the status report during the subsequent preparation of the 6-month interim and 2-month interim status reports were overseen by Robert Forsyth and Dr. Gerald Mackie, COSEWIC Molluscs Specialist Subcommittee Co-Chair. For additional copies contact: COSEWIC Secretariat c/o Canadian Wildlife Service Environment Canada Ottawa, ON K1A 0H3 Tel.: 819-953-3215 Fax: 819-994-3684 E-mail: COSEWIC/[email protected] http://www.cosewic.gc.ca Également disponible en français sous le titre Ếvaluation et Rapport de situation du COSEPAC sur l’huître plate du Pacifique (Ostrea lurida) au Canada. -

Freshwater Mussels of Maritime Canada: a Flashcard Guide
Freshwater Mussels of Maritime Canada: A Flashcard Guide In Wolastoqey, Mi’kmaw, French and English UMBO DORSAL MARGIN ANTERIOR POSTERIOR MARGIN MARGIN VENTRAL MARGIN Donald F. McAlpine, Mary C. Sollows, Jacqueline B. Madill and André L. Martel ISBN 978-0-919326-80-4 All photos copyright the Canadian Museum of Nature Acknowledgements: Funding for this publication provided by the Department of Fisheries and Oceans and the New Brunswick Museum. Special thanks to Ree Brennin Houston, Department of Fisheries and Oceans; Anne Hamilton, Brent Suttie, New Brunswick Archaeological Services Branch, and indigenous language translators Allan Tremblay (Wolastoqiyk), George Paul, Howard Augustine, and Karen Narvey (Mi’kmaw). Citation: McAlpine, D.F., M.C. Sollows, J. B. Madill, and A. L. Martel. 2018. Freshwater Mussels of Maritime Canada: A Flashcard Guide in Wolastoqey, Mi’kmaw, French and English. New Brunswick Museum, Saint John, New Brunswick, and Canadian Museum of Nature, Ottawa, Canada. Use in conjunction with Martel, A. L.,D.F. McAlpine, J. Madill, D. Sabine, A. Paquet, M. Pulsifer and M. Elderkin. 2010. Pp. 551-598. Freshwater Mussels (Bivalvia: Margaritiferidae, Unionidae) of the Atlantic Maritime Ecozone. In D.F. McAlpine and I.M. Smith (eds.). Assessment of Species Diversity in the Atlantic Maritime Ecozone. NRC Research Press, National Research Council of Canada, Ottawa, ON. 785 pp. Nedeau, E.J., M.A. McCollough, and B.I. Swartz. 2000. Freshwater Mussels of Maine. Maine Department of Inland Fisheries and Wildlife, Augusta, ME, 118 pp. -

Pleistocene Molluscs from the Namaqualand Coast
ANNALS OF THE SOUTH AFRICAN MUSEUM ANNALE VAN DIE SUID-AFRIKAANSE MUSEUM Volume 52 Band July 1969 Julie Part 9 Dee! PLEISTOCENE MOLLUSCS FROM THE NAMAQUALAND COAST By A.J.CARRINGTON & B.F.KENSLEY are issued in parts at irregular intervals as material becomes available Obtainable from the South African Museum, P.O. Box 61, Cape Town word uitgegee in dele opongereelde tye na beskikbaarheid van stof OUT OF PRINT/UIT nRUK I, 2(1, 3, 5, 7-8), 3(1-2, 5, t.-p.i.), 5(2, 5, 7-9), 6(1, t.-p.i.), 7(1, 3), 8, 9(1-2), 10(1-3), 11(1-2, 7, t.-p.i.), 21, 24(2), 27, 31(1-3), 38, 44(4)· Price of this part/Prys van hierdie deel Rg.oo Trustees of the South African Museum © 1969 Printed in South Africa by In Suid-Afrika gedruk deur The Rustica Press, Pty., Ltd. Die Rustica-pers, Edms., Bpk. Court Road, Wynberg, Cape Courtweg, Wynberg, Kaap By A. ]. CARRINGTON & B. F. KENSLEY South African Museum, Cape Town (With plates 18 to 29 and I I figures) PAGE Introduction 189 Succession 190 Systematic discussion. 191 Acknowledgements 222 Summary. 222 References 223 INTRODUCTION In the course of an examination of the Tertiary to Recent sediments of the Namaqualand coast, being carried out by one of the authors (A.].C.), a collection of fossil molluscs was assembled from the Pleistocene horizons encountered in the area. The purpose of this paper is to introduce and describe some twenty species from this collection, including forms new to the South Mrican palaeontological literature. -

Missouri's Freshwater Mussels
Missouri mussel invaders Two exotic freshwater mussels, the Asian clam (Corbicula and can reproduce at a much faster rate than native mussels. MISSOURI’S fluminea) and the zebra mussel (Dreissena polymorpha), have Zebra mussels attach to any solid surface, including industrial found their way to Missouri. The Asian clam was introduced pipes, native mussels and snails and other zebra mussels. They into the western U.S. from Asia in the 1930s and quickly spread form dense clumps that suffocate and kill native mussels by eastward. Since 1968 it has spread rapidly throughout Missouri restricting feeding, breathing and other life functions. Freshwater and is most abundant in streams south of the Missouri River. In You can help stop the spread of these mussels by not moving the mid-1980s, zebra mussels hitched a ride in the ballast waters bait or boat well water from one stream to another; dump and of freighter ships traveling from Asia to the Great Lakes. They drain on the ground before leaving. Check all surfaces of your have rapidly moved into the Mississippi River basin and boat and trailer for zebra mussels and destroy them, along with westward to Oklahoma. vegetation caught on the boat or trailer. Wash with hot (104˚F) Asian clam and zebra mussel larvae have an advantage here water at a carwash and allow all surfaces to dry in the sun for at because they don’t require a fish host to reach a juvenile stage least five days before boating again. MusselsMusselsSue Bruenderman, Janet Sternburg and Chris Barnhart Zebra mussels attached to a native mussel JIM RATHERT ZEBRA CHRIS BARNHART ASIAN CLAM MUSSEL Shells are very common statewide in rivers, ponds and reservoirs A female can produce more than a million larvae at one time, and are often found on banks and gravel bars. -

Annex C Water Quality and Heavy Metals in Freshwater Pearl Mussels and Their Habitat
ANNEX C Annex C Water quality and heavy metals in freshwater pearl mussels and their habitat Aspholm, Paul Erik1 , Veersalu, Aune2, Nilsson, Lars Ola1, Larsen, Björn Mejdell3, Christensen, Guttorm4, Olofsson, Patrik5 1 Bioforsk – Norwegian Institute for Agricultural and Environmental Research, Soil and environment Svanhovd, Norway 2 Metsähallitus, Natural Heritage Services Lapland, Finland 3 NINA – Norwegian Institute for nature research, Norway 4 Akvaplan-niva, High North Research Centre, Norway 5 County Administrative Board of Norrbotten, Water and Fisheries Unit, Sweden 1 Background stand what could be the human impacts and Each river is unique in respect to water qualities. effect of these impacts together with the various In general, hydro-chemical conditions in rivers other parameters influencing water quality in are determined by several environmental vari- the individual rivers. Subsequently, it is also ables such as geological conditions, topology, important to know whether any measures might vegetation, climatic conditions and anthropo- result in side-effects or combination effects that genic activities will also differ as regards spatial are also harmful to the mussels. This is especially scales – along the river, and time scales – within important in a long-term perspective. and between years. The habitat changes may occur as a result of In general, the chemistry of northern relatively modest climatic changes (Hastie et al European waters varies considerably, although 2003) and as well the physical microhabitat of nutrient levels are generally low throughout the the freshwater pearl mussel (Hastie et al 2000). northern region of interest in this project e.g. Geist and Auerswald (2007) points out that the due to low temperatures and corresponding stream bed appears to be the most important relatively low biological activities.