Experimental Investigation of Silvering in Late Roman Coinage
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
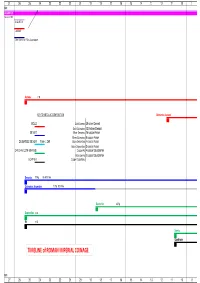
TIMELINE of ROMAN IMPERIAL COINAGE
27 26 25 24 23 22 21 20 19 18 17 16 15 14 13 12 11 10 9 B.C. AUGUSTUS 16 Jan 27 BC AUGUSTUS CAESAR Other title: e.g. Filius Augustorum Aureus 7.8g KEY TO METALLIC COMPOSITION Quinarius Aureus GOLD Gold Aureus 25 silver Denarii Gold Quinarius 12.5 silver Denarii SILVER Silver Denarius 16 copper Asses Silver Quinarius 8 copper Asses DE-BASED SILVER from c. 260 Brass Sestertius 4 copper Asses Brass Dupondius 2 copper Asses ORICHALCUM (BRASS) Copper As 4 copper Quadrantes Brass Semis 2 copper Quadrantes COPPER Copper Quadrans Denarius 3.79g 96-98% fine Quinarius Argenteus 1.73g 92% fine Sestertius 25.5g Dupondius 12.5g As 10.5g Semis Quadrans TIMELINE of ROMAN IMPERIAL COINAGE B.C. 27 26 25 24 23 22 21 20 19 18 17 16 15 14 13 12 11 10 9 8 7 6 5 4 3 2 1 1 2 3 4 5 6 7 8 9 10 11 A.D.A.D. denominational relationships relationships based on Aureus Aureus 7.8g 1 Quinarius Aureus 3.89g 2 Denarius 3.79g 25 50 Sestertius 25.4g 100 Dupondius 12.4g 200 As 10.5g 400 Semis 4.59g 800 Quadrans 3.61g 1600 8 7 6 5 4 3 2 1 1 2 3 4 5 6 7 8 91011 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 19 Aug TIBERIUS TIBERIUS Aureus 7.75g Aureus Quinarius Aureus 3.87g Quinarius Aureus Denarius 3.76g 96-98% fine Denarius Sestertius 27g Sestertius Dupondius 14.5g Dupondius As 10.9g As Semis Quadrans 3.61g Quadrans 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 TIBERIUS CALIGULA CLAUDIUS Aureus 7.75g 7.63g Quinarius Aureus 3.87g 3.85g Denarius 3.76g 96-98% fine 3.75g 98% fine Sestertius 27g 28.7g -

Silvering and Re-Silvering Mirrors & How to Make Your Own One-Way Mirror Solution No
Silvering and Re-Silvering Mirrors & How to Make Your Own One-way Mirror Solution No. 1: Nitrate of Silver (pure) . 40 grains Nitrate of Silver (pure) . 32 grains Distilled Water . 1 pint Ammonia, 26% . To be used as directed. Take one pint of distilled water, pour 4 ounces of this into a glass, and into this put 40 grains of Nitrate of Silver. Dissolve the Nitrate of Silver thoroughly by stirring the water with glass strip (no spoon, or stick, or metal should be used). When it is all thoroughly dissolved, take your medicine dropper and drop 26% Ammonia Water into it one drop at a time; at first it will turn dark; keep dropping the ammonia until it becomes clear again, which will generally take about thirty drops; stopping the addition as soon as it clears. Very often after dropping 30 drops of Ammonia, it does not clear. In that case stir the solution slowly with your left hand and continue dropping the ammonia with the hand, one drop at a time until it does clear, which it will generally do after dropping a few more times. If after dropping seven drops more it does not clear (which takes 37 drops in all) do not drop any more Ammonia, as you are apt to spoil the solution. Then add 32 grains of the Nitrate of Silver, additional. Dissolve by stirring with your glass strip. When it is all dissolved, pour the mixture back into the pint of water first measured out. Let it stand for one hour or more to allow the sediment to settle on the bottom. -

Biography: Justus Von Liebig
Biography: Justus von Liebig Justus von Liebig (1803 – 1873) was a German chemist. He taught chemistry at the University of Giessen and the University of Munich. The University of Giessen currently bears his name. Liebig is called the father of fertilizers. He confirmed the hypothesis concerning the mineral nutrition of plants, which became the basis for the development of modern agricultural chemistry. Liebig’s research is considered a precursor to the study of the impact of environmental factors on organisms. He formulated the law of the minimum, which states that the scarcest resource is what limits a given organism. He also developed a process for producing meat extract and founded the company Liebig Extract of Meat Company whose trademark was the beef bouillon cube, which he invented. Justus von Liebig was born into a middle class In 1824, at the age of 21, Liebig became a family from Darmstadt on May 12, 1803. As a professor at the University of Giessen. While in child, he was already fascinated by chemistry. Germany, he founded and edited the magazine When he was 13 years old, most of the crops in the Annalen der Chemie, which became the leading Northern Hemisphere were destroyed by a journal of chemistry in Germany. volcanic winter. Germans were among the most In 1837, he was elected a member of the Royal affected. It is said that this experience influenced Swedish Academy of Sciences, and in 1845, started the subsequent work of Liebig and the working at the University of Munich, where he establishment of his company. remained until his death. -

Currency, Bullion and Accounts. Monetary Modes in the Roman World
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Ghent University Academic Bibliography 1 Currency, bullion and accounts. Monetary modes in the Roman world Koenraad Verboven in Belgisch Tijdschrift voor Numismatiek en Zegelkunde/ Revue Belge de Numismatique et de Sigillographie 155 (2009) 91-121 Finley‟s assertion that “money was essentially coined metal and nothing else” still enjoys wide support from scholars1. The problems with this view have often been noted. Coins were only available in a limited supply and large payments could not be carried out with any convenience. Travelling with large sums in coins posed both practical and security problems. To quote just one often cited example, Cicero‟s purchase of his house on the Palatine Hill for 3.5 million sesterces would have required 3.4 tons of silver denarii2. Various solutions have been proposed : payments in kind or by means of bullion, bank money, transfer of debt notes or sale credit. Most of these combine a functional view of money („money is what money does‟) with the basic belief that coinage in the ancient world was the sole dominant monetary instrument, with others remaining „second-best‟ alternatives3. Starting from such premises, research has focused on identifying and assessing the possible alternative instruments to effectuate payments. Typical research questions are for instance the commonness (or not) of giro payments, the development (or underdevelopment) of financial instruments, the monetary nature (or not) of ancient debt notes, the commonness (or not) of payments in kind, and so forth. Despite Ghent University, Department of History 1 M. -

Coins and Medals Including Renaissance and Later Medals from the Collection of Dr Charles Avery and Byzantine Coins from the Estate of Carroll F
Coins and Medals including Renaissance and Later Medals from the Collection of Dr Charles Avery and Byzantine Coins from the Estate of Carroll F. Wales (Part I) To be sold by auction at: Sotheby’s, in the Upper Grosvenor Gallery The Aeolian Hall, Bloomfield Place New Bond Street London W1 Days of Sale: Wednesday 11 and Thursday 12 June 2008 10.00 am and 2.00 pm Public viewing: 45 Maddox Street, London W1S 2PE Friday 6 June 10.00 am to 4.30 pm Monday 9 June 10.00 am to 4.30 pm Tuesday 10 June 10.00 am to 4.30 pm Or by previous appointment. Catalogue no. 31 Price £10 Enquiries: James Morton, Tom Eden, Paul Wood, Jeremy Cheek or Stephen Lloyd Cover illustrations: Lot 465 (front); Lot 1075 (back); Lot 515 (inside front and back covers, all at two-thirds actual size) in association with 45 Maddox Street, London W1S 2PE Tel.: +44 (0)20 7493 5344 Fax: +44 (0)20 7495 6325 Email: [email protected] Website: www.mortonandeden.com This auction is conducted by Morton & Eden Ltd. in accordance with our Conditions of Business printed at the back of this catalogue. All questions and comments relating to the operation of this sale or to its content should be addressed to Morton & Eden Ltd. and not to Sotheby’s. Important Information for Buyers All lots are offered subject to Morton & Eden Ltd.’s Conditions of Business and to reserves. Estimates are published as a guide only and are subject to review. The actual hammer price of a lot may well be higher or lower than the range of figures given and there are no fixed “starting prices”. -

Small Change and Big Changes: Minting and Money After the Fall of Rome
This is a repository copy of Small Change and Big Changes: minting and money after the Fall of Rome. White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/90089/ Version: Draft Version Other: Jarrett, JA (Completed: 2015) Small Change and Big Changes: minting and money after the Fall of Rome. UNSPECIFIED. (Unpublished) Reuse Unless indicated otherwise, fulltext items are protected by copyright with all rights reserved. The copyright exception in section 29 of the Copyright, Designs and Patents Act 1988 allows the making of a single copy solely for the purpose of non-commercial research or private study within the limits of fair dealing. The publisher or other rights-holder may allow further reproduction and re-use of this version - refer to the White Rose Research Online record for this item. Where records identify the publisher as the copyright holder, users can verify any specific terms of use on the publisher’s website. Takedown If you consider content in White Rose Research Online to be in breach of UK law, please notify us by emailing [email protected] including the URL of the record and the reason for the withdrawal request. [email protected] https://eprints.whiterose.ac.uk/ Small Change and Big Changes: It’s a real pleasure to be back at the Barber again so soon, and I want to start by thanking Nicola, Robert and Jen for letting me get away with scheduling myself like this as a guest lecturer for my own exhibition. I’m conscious that in speaking here today I’m treading in the footsteps of some very notable numismatists, but right now the historic name that rings most loudly in my consciousness is that of the former Curator of the coin collection here and then Lecturer in Numismatics in the University, Michael Hendy. -

A Handbook of Greek and Roman Coins
CORNELL UNIVERSITY LIBRARY BOUGHT WITH THE INCOME OF THE SAGE ENDOWMENT FUND GIVEN IN 1891 BY HENRY WILLIAMS SAGE Cornell University Library CJ 237.H64 A handbook of Greek and Roman coins. 3 1924 021 438 399 Cornell University Library The original of this book is in the Cornell University Library. There are no known copyright restrictions in the United States on the use of the text. http://www.archive.org/details/cu31924021438399 f^antilioofcs of glrcfjaeologj) anU Antiquities A HANDBOOK OF GREEK AND ROMAN COINS A HANDBOOK OF GREEK AND ROMAN COINS G. F. HILL, M.A. OF THE DEPARTMENT OF COINS AND MEDALS IN' THE bRITISH MUSEUM WITH FIFTEEN COLLOTYPE PLATES Hon&on MACMILLAN AND CO., Limited NEW YORK: THE MACMILLAN COMPANY l8 99 \_All rights reserved'] ©jcforb HORACE HART, PRINTER TO THE UNIVERSITY PREFACE The attempt has often been made to condense into a small volume all that is necessary for a beginner in numismatics or a young collector of coins. But success has been less frequent, because the knowledge of coins is essentially a knowledge of details, and small treatises are apt to be un- readable when they contain too many references to particular coins, and unprofltably vague when such references are avoided. I cannot hope that I have passed safely between these two dangers ; indeed, my desire has been to avoid the second at all risk of encountering the former. At the same time it may be said that this book is not meant for the collector who desires only to identify the coins which he happens to possess, while caring little for the wider problems of history, art, mythology, and religion, to which coins sometimes furnish the only key. -

Downloaded on 2019-04-30T23:20:12Z
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Cork Open Research Archive UCC Library and UCC researchers have made this item openly available. Please let us know how this has helped you. Thanks! Title Constantine I and a new Christian golden age: a secretly Christian reverse type identified? Author(s) Woods, David Publication date 2018-09 Original citation Woods, David (2018) 'Constantine I and a New Christian Golden Age: A Secretly Christian Reverse Type Identified?' Greek, Roman and Byzantine Studies, 58, pp. 366-388. Type of publication Article (peer-reviewed) Link to publisher's https://grbs.library.duke.edu/article/view/16063 version Access to the full text of the published version may require a subscription. Rights © 2018 David Woods. http://creativecommons.org/licenses/by/3.0/ Item downloaded http://hdl.handle.net/10468/7749 from Downloaded on 2019-04-30T23:20:12Z Constantine I and a New Christian Golden Age: A Secretly Christian Reverse Type Identified? David Woods HE PURPOSE of this paper is to explore the significance of a reverse type used on solidi struck in the name of T Constantine I (306–337) alone at the mints of Nico- media, Sirmium, Ticinum, and Trier during his vicennial year starting on 26 July 325.1 This type depicts what is usually described as two interlaced wreaths surrounded by the legend CONSTANTINVS AVG ( fig. 1).2 With the exception of the issue from Trier, Constantine used this type as part of the coinage struck for donatives as he stopped in the various mint-towns during the course of his journey from Nicomedia to Rome.3 The only minor variation between the mints is that the coins from Sirmium, Ticinum, and Trier always depict a single star cen- 1 The standard catalogue of the coinage of Constantine I remains Patrick M. -

Byzantine Coinage
BYZANTINE COINAGE Philip Grierson Dumbarton Oaks Research Library and Collection Washington, D.C. © 1999 Dumbarton Oaks Trustees for Harvard University Washington, D.C. All rights reserved Printed in the United States of America Second Edition Cover illustrations: Solidus of Justinian II (enlarged 5:1) ISBN 0-88402-274-9 Preface his publication essentially consists of two parts. The first part is a second Tedition of Byzantine Coinage, originally published in 1982 as number 4 in the series Dumbarton Oaks Byzantine Collection Publications. Although the format has been slightly changed, the content is fundamentally the same. The numbering of the illustrations,* however, is sometimes different, and the text has been revised and expanded, largely on the advice and with the help of Cécile Morrisson, who has succeeded me at Dumbarton Oaks as advisor for Byzantine numismatics. Additions complementing this section are tables of val- ues at different periods in the empire’s history, a list of Byzantine emperors, and a glossary. The second part of the publication reproduces, in an updated and slightly shorter form, a note contributed in 1993 to the International Numismatic Commission as one of a series of articles in the commission’s Compte-rendus sketching the histories of the great coin cabinets of the world. Its appearance in such a series explains why it is written in the third person and not in the first. It is a condensation of a much longer unpublished typescript, produced for the Coin Room at Dumbarton Oaks, describing the formation of the collection and its publication. * The coins illustrated are in the Dumbarton Oaks and Whittemore collections and are re- produced actual size unless otherwise indicated. -

Roman Coins Elementary Manual
^1 If5*« ^IP _\i * K -- ' t| Wk '^ ^. 1 Digitized by Google Digitized by Google Digitized by Google Digitized by Google Digitized by Google Digitized by Google PROTAT BROTHERS, PRINTBRS, MACON (PRANCi) Digitized by Google ROMAN COINS ELEMENTARY MANUAL COMPILED BY CAV. FRANCESCO gNECCHI VICE-PRBSIDENT OF THE ITALIAN NUMISMATIC SOaETT, HONORARY MEMBER OF THE LONDON, BELGIAN AND SWISS NUMISMATIC SOCIBTIES. 2"^ EDITION RKVISRD, CORRECTED AND AMPLIFIED Translated by the Rev<> Alfred Watson HANDS MEMBF,R OP THE LONDON NUMISMATIC SOCIETT LONDON SPINK & SON 17 & l8 PICCADILLY W. — I & 2 GRACECHURCH ST. B.C. 1903 (ALL RIGHTS RF^ERVED) Digitized by Google Arc //-/7^. K.^ Digitized by Google ROMAN COINS ELEMENTARY MANUAL AUTHOR S PREFACE TO THE ENGLISH EDITION In the month of July 1898 the Rev. A. W. Hands, with whom I had become acquainted through our common interests and stud- ieSy wrote to me asking whether it would be agreeable to me and reasonable to translate and publish in English my little manual of the Roman Coinage, and most kindly offering to assist me, if my knowledge of the English language was not sufficient. Feeling honoured by the request, and happy indeed to give any assistance I could in rendering this science popular in other coun- tries as well as my own, I suggested that it would he probably less trouble ii he would undertake the translation himselt; and it was with much pleasure and thankfulness that I found this proposal was accepted. It happened that the first edition of my Manual was then nearly exhausted, and by waiting a short time I should be able to offer to the English reader the translation of the second edition, which was being rapidly prepared with additions and improvements. -

The Gender of Money: Byzantine Empresses on Coins (324–802)’ Gender & History, Vol.12 No
Gender & History ISSN 0953–5233 Leslie Brubaker and Helen Tobler, ‘The Gender of Money: Byzantine Empresses on Coins (324–802)’ Gender & History, Vol.12 No. 3 November 2000, pp. 572–594. The Gender of Money: Byzantine Empresses on Coins (324–802) Leslie Brubaker and Helen Tobler Coins played different roles in the ancient and medieval worlds from those that they play in the economy today. In the late antique and early Byzantine world – that is, roughly between 300 and 800 – there were in a sense two currencies: gold coins and base metal (copper) coins. Both were minted and distributed by the state, but the gold solidi (in Latin) or nomismata (in Greek), introduced in 309, were by the end of the fifth century in practice used above all for the payment of tax and for major transactions such as land sales, while the copper coins (nummi, replaced in 498 by folles) were broadly the currency of market transactions.1 Another striking difference is that late antique and Byzantine coin types changed with great frequency: as an extreme example, Maria Alföldi catalogued over seven hundred different types for a single emperor, Constantine I the Great (306–37, sole ruler from 324).2 There are many reasons for this, but one of the most import- ant has to do with communication: centuries before the advent of the press, images on coins were a means to circulate information about the state. This is particularly true of the first three and a half centuries covered by this article. While the extent to which coins were used in daily exchange transactions is still uncertain, and was very variable, the frequency with which they appear in archaeological excavations of urban sites throughout the former eastern Roman empire until 658 indicates their wide diffusion. -

Historic Mercury Amalgam Mirrors: History, Safety and Preservation by Kathleen Payne De Chavez
Tech Notes, Spring 2010 Historic Mercury Amalgam Mirrors: History, Safety and Preservation By Kathleen Payne de Chavez Large mirror-plates are now the indispensable ornaments of every large and sumptuous apartment; they diffuse luster and gayety round them, by reflecting the rays of light in a thousands lines, and by multiplying indefinitely the images of objects placed between opposite parallel planes. —Ure’s Dictionary (1856) History While ancient civilizations, including the Romans, Mayans, and Egyptians, employed highly polished metal discs as mirrors, what we understand as a modern mirror, a glass with a reflective metal-foil backing, came into being sometime around the end of the 15th century. In Venice the development of cristallo, a transparent, colorless glass, gave Venetian glassmakers an advantage in the creation of high-quality mirrors with clear reflectance. Initially, some glassmakers poured a mirroring mixture of lead and antimony onto the surface of highly polished blown-glass plates, but this method yielded a rough surface with dim reflection. In the early 16th century, the Del Gallo glassmakers of the Venetian island of Murano improved the method by developing a mercury-tin amalgam technique for mirroring the glass surface. T his method deposited a thin layer of tin on the surface creating unparalleled reflectance. So revolutionary was this technique that the Republic of Venice forbade Muranese glassmakers from emigrating and taking their trade secrets to other regions. However, by the mid-seventeenth century some of these skilled craftsmen had escaped and brought the trade to France, and from there, the world. Despite the appearance of a competing mirror-making process, mercury-tin amalgam remained the predominant form of mirroring through the 19th century.