Electroplating with Manufacture of Electrochemicals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

126 Metal Products
Lake Tuggeranong College LEARN – THRIVE - CONNECT Metal Products A/M Working with metal has fascinated people for centuries. Metal forming processes have advanced significantly in recent times and have enabled the creation of objects which have changed the world forever. These processes are now able to be experienced by students through the development of metal fabrication projects which provide an understanding of metal properties and construction techniques. The considerable time devoted to the practical skills in this course makes it an ideal choice for students who are looking for a unique practical learning environment. Rationale Why would you do this course? Working with metal is an enjoyable activity which more students need to experience! Being able to apply metal fabricating processes to create projects with a student design input is a major emphasis in the course. The range of fundamental skills employed in the metal fabrication industry readily engage students in their course work. There is a current skills shortage in the metal trade areas as evidenced by the inclusion of Metal Fabricator and Welder in the National Skills Needs list. There is a federal government push for apprenticeships using the skills learnt during this course. Beyond the classroom, this subject offers you: • Pathways to metal trades: boiler maker/metal fabricator • Skills that are interlinked between other trades: construction, plumber, electrician • Basic handyman skills Learner dispositions What type of person usually studies this course? Learners who would study this course typically achieve a satisfaction from being able to create practical objects using techniques such as welding, machining, plasma cutting, sheet metal forming and more. -

Silvering and Re-Silvering Mirrors & How to Make Your Own One-Way Mirror Solution No
Silvering and Re-Silvering Mirrors & How to Make Your Own One-way Mirror Solution No. 1: Nitrate of Silver (pure) . 40 grains Nitrate of Silver (pure) . 32 grains Distilled Water . 1 pint Ammonia, 26% . To be used as directed. Take one pint of distilled water, pour 4 ounces of this into a glass, and into this put 40 grains of Nitrate of Silver. Dissolve the Nitrate of Silver thoroughly by stirring the water with glass strip (no spoon, or stick, or metal should be used). When it is all thoroughly dissolved, take your medicine dropper and drop 26% Ammonia Water into it one drop at a time; at first it will turn dark; keep dropping the ammonia until it becomes clear again, which will generally take about thirty drops; stopping the addition as soon as it clears. Very often after dropping 30 drops of Ammonia, it does not clear. In that case stir the solution slowly with your left hand and continue dropping the ammonia with the hand, one drop at a time until it does clear, which it will generally do after dropping a few more times. If after dropping seven drops more it does not clear (which takes 37 drops in all) do not drop any more Ammonia, as you are apt to spoil the solution. Then add 32 grains of the Nitrate of Silver, additional. Dissolve by stirring with your glass strip. When it is all dissolved, pour the mixture back into the pint of water first measured out. Let it stand for one hour or more to allow the sediment to settle on the bottom. -

Updated Course Guide Jan 2017.Pdf
1 Wilmot Union High School Mission Statement As a professional learning community, Wilmot Union High School's core purpose is to ensure our students are college, career, and civic ready by fostering a culture of life-long learning. District Vision As a learning community and through community involvement, Wilmot Union High School has developed a clear sense of who we want to become through a process where district stakeholders: students, staff, parents, community members, business partners and Board of Education members came together and identified the key characteristics and values we want to exemplify. These characteristics and values were aligned under five pillars to further define the key areas of focus for our learning community. I. Safe and Supportive Learning Environment II. Equity and Access for All Students III. Community Partnerships IV. Collaborative Culture for Learning V. Curriculum, Instruction and Assessment It is through these five pillars and their guiding statements that WUHS and our stakeholders will empower one another to create and fulfill the goals and commitments that will bring our vision to fruition. We invite every stakeholder of the WUHS learning community to enter in to this process of adopting the defined values to fulfill our vision of an exemplary school. 2 Wilmot Union High School P.O. Box 8 11112 – 308th Avenue Wilmot, WI 53192 High School Administration (262) 862-2351 John LaFleur, Ph.D., Principal [email protected] Tom Blair, Associate Principal [email protected] Luke Braden, Associate -

Biography: Justus Von Liebig
Biography: Justus von Liebig Justus von Liebig (1803 – 1873) was a German chemist. He taught chemistry at the University of Giessen and the University of Munich. The University of Giessen currently bears his name. Liebig is called the father of fertilizers. He confirmed the hypothesis concerning the mineral nutrition of plants, which became the basis for the development of modern agricultural chemistry. Liebig’s research is considered a precursor to the study of the impact of environmental factors on organisms. He formulated the law of the minimum, which states that the scarcest resource is what limits a given organism. He also developed a process for producing meat extract and founded the company Liebig Extract of Meat Company whose trademark was the beef bouillon cube, which he invented. Justus von Liebig was born into a middle class In 1824, at the age of 21, Liebig became a family from Darmstadt on May 12, 1803. As a professor at the University of Giessen. While in child, he was already fascinated by chemistry. Germany, he founded and edited the magazine When he was 13 years old, most of the crops in the Annalen der Chemie, which became the leading Northern Hemisphere were destroyed by a journal of chemistry in Germany. volcanic winter. Germans were among the most In 1837, he was elected a member of the Royal affected. It is said that this experience influenced Swedish Academy of Sciences, and in 1845, started the subsequent work of Liebig and the working at the University of Munich, where he establishment of his company. remained until his death. -

Design of Forming Processes: Bulk Forming
1 Design of Forming Processes: Bulk Forming Chester J. Van Tyne Colorado School of Mines, Golden, Colorado, U.S.A. I. BULK DEFORMATION atures relative to the melting point of the metal. Hot working occurs at temperatures above tJllerecrystalliza- Bulk defonnation is a metal-fonning process where the tion temperature of the metal. There is a third temper- defonnation is three-dimensional in nature. The pri- ature range, warm working, which is being critically mary use of the tenn bulk deformation is to distinguish it examined due to energy savings and is, in some cases, from sheet-fonning processes. In sheet-forming opera- used by industries. tions, the defonnation stressesare usually in the plane of the sheet metal, whereas in bulk defonnation, the 1. Cold Working Temperatures defonnation stresses possess components in all three Cold working usually refers to metal deformation that is coordinate directions. Bulk defonnation includes metal carried out at room temperature. Th,~ phenomenon working processes such as forging, extrusion, rolling, associated with cold work occurs wht:n the metal is and drawing. deformed at temperatures that are about 30% or less of its melting temperature on an absolute temperature scale. During cold work, the metal ,~xperiences an II. CLASSIFICATION OF DEFORMATION increased number of dislocations and elltanglement of PROCESSES these dislocations, causing strain hardening. With strain hardening, the strength of the metal increases with The classification of deformation processescan be done deformation. To recrystallize the metal, ;i thermal treat- in one of several ways. The more common classification ment, called an anneal, is often needed. During anneal- schemes are based on temperature, flow behavior, and ing, the strength of the metal can be drastically reduced stressstate. -

Introduction to Metal Forming Operations
Introduction to Metal Forming Operations IME 340/240 Classification of Forming Processes • There are a number of ways to divide up forming processes – Hot working, warm working, cold working – Bulk forming, sheet metal forming – Primary and component producing processes – Steady and non-steady processes – Continuous and incremental forming processes Classification of Forming Processes • Cold working – Temperature < 0.3 * melting point in deg. K – In practice for most engineering metal this means room temperature – Work hardening is dominant • Hot working – Above the recrystalization temperature – Temperature > 0.5 (or 0.6) * melting point in deg. K – Strain rate sensitivity more important • Warm working – Temperature between 0.3 and 0.5 of melting point – Flow stresses somewhat less than cold working Classification of Forming Processes • Sheet metal forming – Input material in sheet form – Thickness changes very small – Stress systems largely tensile • Bulk forming – Input material in the form of bars, billets, etc. – Thickness of material usually substantially reduced – Stress systems largely compressive Classification of Forming Processes • Primary forming processes – Processes predominantly for producing materials for further processing – Examples are rolling, drawing, extrusion, etc. • Component producing processes – Processes for producing component parts – Input materials produced by primary processes – Examples are forging, deep drawing, stretch forming, etc. Classification of Forming Processes • Steady state forming processes – -

Experimental Investigation of Silvering in Late Roman Coinage
Mat. Res. Soc. Symp. Proc. Vol. 712 © 2002 Materials Research Society Experimental investigation of silvering in late Roman coinage C. Vlachou, J.G. McDonnell, R.C. Janaway Department of Archaeological Sciences, University of Bradford, BD7 1DP, UK. ABSTRACT Roman Coinage suffered from severe debasement during the 3rd century AD. By 250 AD., the production of complex copper alloy (Cu-Sn-Pb-Ag) coins with a silvered surface, became common practice. The same method continued to be applied during the 4th century AD for the production of a new denomination introduced by Diocletian in 293/4 AD. Previous analyses of these coins did not solve key technological issues and in particular, the silvering process. The British Museum kindly allowed further research at Bradford to examine coins from Cope’s Archive in more detail, utilizing XRF, SEM-EDS metallography, LA-ICP-MS and EPMA. Metallographic and SEM examination of 128 coins, revealed that the silver layer was very difficult to trace because its thickness was a few microns and in some cases it was present under the corrosion layer. Results derived from the LA-ICP-MS and EPMA analyses have demonstrated, for the first time, the presence of Hg in the surface layers of these coins. A review of ancient sources and historic literature indicated possible methods which might have been used for the production of the plating. A programme of plating experiments was undertaken to examine a number of variables in the process, such as amalgam preparation, and heating cycles. Results from the experimental work are presented. ITRODUCTION Coinage in the Late Roman Period suffered from severe debasement. -

PDH Course M381
PDHonline Course M 497 (6 PDH) _______________________________________________________________________________________ Conventional Machining Technology Fundamentals Instructor: Jurandir Primo, PE 2013 PDH Online | PDH Center 5272 Meadow Estates Drive Fairfax, VA 22030-6658 Phone & Fax: 703-988-0088 www.PDHonline.org www.PDHcenter.com An Approved Continuing Education Provider www.PDHcenter.com PDH Course M 497 www.PDHonline.org CONVENTIONAL MACHINING TECHNOLOGY – FUNDAMENTALS Introduction Shaping Machines Lathes Slotting Machines - Metalworking lathes - Planing, shaping and slotting calculations - Classification of lathes - Turning operations Boring Machines - Semiautomatic and automatic lathes - Types of boring machines - Accessories - Boring types - Live centers and dead centers - Boring calculations - Rests and micrometer supports - Lathe cutting tools Hobbing & Gear Shaping Machines - Lathe calculations - Common gear generation types - Graduate micrometer and measurements - Details of involute gearing - Tools and inserts - Proper meshing and contact ratio - Common holders with inserts - Gear Shaping Machines - Goose-neck holders with inserts Broaching Machines Drilling Machines - Horizontal broaching machines - Classification of drilling machines - Vertical broaching machines - Application of drilling machines - Broaching principles - Types of drills - Broaching configuration - Drill sizes and geometry - Materials of broaches - Drill point angles - Geometry of broaching teeth - Drill holding & clamping of workpieces - Broaching operations -

Manufacturing Processes by H.N. Gupta.Pdf
This page intentionally left blank MANUFACTURING PROCESSES (SECOND EDITION) H.N. Gupta B.Sc., G.I. Mech.E (London), FIE Visiting Professor Department of Mechanical Engineering I.E.T., Lucknow, U.P. Technical University R.C. Gupta B.Sc., B.E., M.Tech., Ph.D. Professor and Head Department of Mechanical Engineering I.E.T., Lucknow, U.P. Technical University Arun Mittal Senior Faculty Department of Mechanical Engineering I.E.T., Lucknow, U.P. Technical University Copyright © 2009, New Age International (P) Ltd., Publishers Published by New Age International (P) Ltd., Publishers All rights reserved. No part of this ebook may be reproduced in any form, by photostat, microfilm, xerography, or any other means, or incorporated into any information retrieval system, electronic or mechanical, without the written permission of the publisher. All inquiries should be emailed to [email protected] ISBN (13) : 978-81-224-2844-5 PUBLISHING FOR ONE WORLD NEW AGE INTERNATIONAL (P) LIMITED, PUBLISHERS 4835/24, Ansari Road, Daryaganj, New Delhi - 110002 Visit us at www.newagepublishers.com Preface to the Second Edition The authors of the book ‘‘Manufacturing Processes’’ are thrilled at the speed with which the first edition of the book has been snapped up and exhausted within four months of its publication necessitating a reprint. This proves that the book has been found useful both by teachers and the students. This is extremely gratifying. It has been felt that to make the text of the book even more useful, certain changes have been made. Therefore the text of the Unit I and Unit IV has been completely rewritten in the second edition of the book. -
Metal Forming Fundamentals
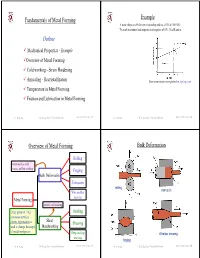
Fundamentals of Metal Forming Example A metal obeys the Hollomon relationship and has a UTS of 300 MPa. To reach maximum load requires an elongation of 35%. Find K and n. Outline 9 Mechanical Properties - Example 9Overview of Metal Forming 9 Cold working - Strain Hardening 9 Annealing - Recrystallization True stress-strain curve plotted on log-log scale 9 Temperature in Metal Forming 9 Friction and Lubrication in Metal Forming Dr. M. Medraj Mech. Eng. Dept. - Concordia University Mech 421/6511 lecture 3/1 Dr. M. Medraj Mech. Eng. Dept. - Concordia University Mech 421/6511 lecture 3/2 Overview of Metal Forming Bulk Deformation Rolling Performed as cold, warm, and hot working Forging Bulk Deformation Extrusion rolling extrusion Wire and bar drawing Metal Forming Mainly cold working Large group of mfg Bending processes in which Sheet plastic deformation is Shearing used to change the shape Metalworking of metal workpieces Deep and cup Wire/bar drawing drawing forging Dr. M. Medraj Mech. Eng. Dept. - Concordia University Mech 421/6511 lecture 3/3 Dr. M. Medraj Mech. Eng. Dept. - Concordia University Mech 421/6511 lecture 3/4 Sheet Metalworking Formability (workability) Formability of the material depends on: (1) process variables - ……………… Desirable material properties in metal forming: - ……………… – Low yield strength and high ductility - ……………… (2) Metallurgical changes during deformation bending Deep/cup drawing - formation of voids, composition, inclusions, precipitation, .... etc. Ductility increases and yield strength decreases when work temperature is raised → Any deformation operation can be accomplished with lower forces and power at elevated temperature shearing Dr. M. Medraj Mech. Eng. Dept. - Concordia University Mech 421/6511 lecture 3/5 Dr. -

Review Article Semisolid Metal Processing Techniques for Nondendritic Feedstock Production
Hindawi Publishing Corporation The Scientific World Journal Volume 2013, Article ID 752175, 16 pages http://dx.doi.org/10.1155/2013/752175 Review Article Semisolid Metal Processing Techniques for Nondendritic Feedstock Production M. N. Mohammed,1 M. Z. Omar,1 M. S. Salleh,1 K. S. Alhawari,1 and P. Kapranos2 1 Department of Mechanical and Materials Engineering, Faculty of Engineering and Built Environment, Universiti Kebangsaan Malaysia (UKM), 43600 Bangi, Selangor, Malaysia 2 Department of Materials Science and Engineering, The University of Sheffield, Sir Robert Hadfield Building, Mappin Street, Sheffield S1 3JD, UK Correspondence should be addressed to M. Z. Omar; [email protected] Received 26 June 2013; Accepted 28 July 2013 Academic Editors: A. G. Magalhaes˜ and A. Tonkikh Copyright © 2013 M. N. Mohammed et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Semisolid metal (SSM) processing or thixoforming is widely known as a technology that involves the formation of metal alloys between solidus and liquidus temperatures. For the procedure to operate successfully, the microstructure of the starting material must consist of solid near-globular grains surrounded by a liquid matrix and a wide solidus-to-liquidus transition area. Currently, this process is industrially successful, generating a variety of products with high quality parts in various industrial sectors. Throughout the years since its inception, a number of technologies to produce the appropriate globular microstructure have been developed and applied worldwide. The main aim of this paper is to classify the presently available SSM technologies and present a comprehensive review of the potential mechanisms that lead to microstructural alterations during the preparation of feedstock materialsforSSMprocessing. -

Historic Mercury Amalgam Mirrors: History, Safety and Preservation by Kathleen Payne De Chavez
Tech Notes, Spring 2010 Historic Mercury Amalgam Mirrors: History, Safety and Preservation By Kathleen Payne de Chavez Large mirror-plates are now the indispensable ornaments of every large and sumptuous apartment; they diffuse luster and gayety round them, by reflecting the rays of light in a thousands lines, and by multiplying indefinitely the images of objects placed between opposite parallel planes. —Ure’s Dictionary (1856) History While ancient civilizations, including the Romans, Mayans, and Egyptians, employed highly polished metal discs as mirrors, what we understand as a modern mirror, a glass with a reflective metal-foil backing, came into being sometime around the end of the 15th century. In Venice the development of cristallo, a transparent, colorless glass, gave Venetian glassmakers an advantage in the creation of high-quality mirrors with clear reflectance. Initially, some glassmakers poured a mirroring mixture of lead and antimony onto the surface of highly polished blown-glass plates, but this method yielded a rough surface with dim reflection. In the early 16th century, the Del Gallo glassmakers of the Venetian island of Murano improved the method by developing a mercury-tin amalgam technique for mirroring the glass surface. T his method deposited a thin layer of tin on the surface creating unparalleled reflectance. So revolutionary was this technique that the Republic of Venice forbade Muranese glassmakers from emigrating and taking their trade secrets to other regions. However, by the mid-seventeenth century some of these skilled craftsmen had escaped and brought the trade to France, and from there, the world. Despite the appearance of a competing mirror-making process, mercury-tin amalgam remained the predominant form of mirroring through the 19th century.