© Copyright 2019 Itzue Wendolin Caviedes Solis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CAT Vertebradosgt CDC CECON USAC 2019
Catálogo de Autoridades Taxonómicas de vertebrados de Guatemala CDC-CECON-USAC 2019 Centro de Datos para la Conservación (CDC) Centro de Estudios Conservacionistas (Cecon) Facultad de Ciencias Químicas y Farmacia Universidad de San Carlos de Guatemala Este documento fue elaborado por el Centro de Datos para la Conservación (CDC) del Centro de Estudios Conservacionistas (Cecon) de la Facultad de Ciencias Químicas y Farmacia de la Universidad de San Carlos de Guatemala. Guatemala, 2019 Textos y edición: Manolo J. García. Zoólogo CDC Primera edición, 2019 Centro de Estudios Conservacionistas (Cecon) de la Facultad de Ciencias Químicas y Farmacia de la Universidad de San Carlos de Guatemala ISBN: 978-9929-570-19-1 Cita sugerida: Centro de Estudios Conservacionistas [Cecon]. (2019). Catálogo de autoridades taxonómicas de vertebrados de Guatemala (Documento técnico). Guatemala: Centro de Datos para la Conservación [CDC], Centro de Estudios Conservacionistas [Cecon], Facultad de Ciencias Químicas y Farmacia, Universidad de San Carlos de Guatemala [Usac]. Índice 1. Presentación ............................................................................................ 4 2. Directrices generales para uso del CAT .............................................. 5 2.1 El grupo objetivo ..................................................................... 5 2.2 Categorías taxonómicas ......................................................... 5 2.3 Nombre de autoridades .......................................................... 5 2.4 Estatus taxonómico -

Sex-Chromosome Evolution of Palearctic Tree Frogs in Space and Time
Unicentre CH-1015 Lausanne http://serval.unil.ch Year : 2015 Sex-chromosome evolution of Palearctic tree frogs in space and time Dufresnes Christophe Dufresnes Christophe, 2015, Sex-chromosome evolution of Palearctic tree frogs in space and time Originally published at : Thesis, University of Lausanne Posted at the University of Lausanne Open Archive http://serval.unil.ch Document URN : urn:nbn:ch:serval-BIB_1F84E46189071 Droits d’auteur L'Université de Lausanne attire expressément l'attention des utilisateurs sur le fait que tous les documents publiés dans l'Archive SERVAL sont protégés par le droit d'auteur, conformément à la loi fédérale sur le droit d'auteur et les droits voisins (LDA). A ce titre, il est indispensable d'obtenir le consentement préalable de l'auteur et/ou de l’éditeur avant toute utilisation d'une oeuvre ou d'une partie d'une oeuvre ne relevant pas d'une utilisation à des fins personnelles au sens de la LDA (art. 19, al. 1 lettre a). A défaut, tout contrevenant s'expose aux sanctions prévues par cette loi. Nous déclinons toute responsabilité en la matière. Copyright The University of Lausanne expressly draws the attention of users to the fact that all documents published in the SERVAL Archive are protected by copyright in accordance with federal law on copyright and similar rights (LDA). Accordingly it is indispensable to obtain prior consent from the author and/or publisher before any use of a work or part of a work for purposes other than personal use within the meaning of LDA (art. 19, para. 1 letter a). -

Amphibian Alliance for Zero Extinction Sites in Chiapas and Oaxaca
Amphibian Alliance for Zero Extinction Sites in Chiapas and Oaxaca John F. Lamoreux, Meghan W. McKnight, and Rodolfo Cabrera Hernandez Occasional Paper of the IUCN Species Survival Commission No. 53 Amphibian Alliance for Zero Extinction Sites in Chiapas and Oaxaca John F. Lamoreux, Meghan W. McKnight, and Rodolfo Cabrera Hernandez Occasional Paper of the IUCN Species Survival Commission No. 53 The designation of geographical entities in this book, and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of IUCN concerning the legal status of any country, territory, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. The views expressed in this publication do not necessarily reflect those of IUCN or other participating organizations. Published by: IUCN, Gland, Switzerland Copyright: © 2015 International Union for Conservation of Nature and Natural Resources Reproduction of this publication for educational or other non-commercial purposes is authorized without prior written permission from the copyright holder provided the source is fully acknowledged. Reproduction of this publication for resale or other commercial purposes is prohibited without prior written permission of the copyright holder. Citation: Lamoreux, J. F., McKnight, M. W., and R. Cabrera Hernandez (2015). Amphibian Alliance for Zero Extinction Sites in Chiapas and Oaxaca. Gland, Switzerland: IUCN. xxiv + 320pp. ISBN: 978-2-8317-1717-3 DOI: 10.2305/IUCN.CH.2015.SSC-OP.53.en Cover photographs: Totontepec landscape; new Plectrohyla species, Ixalotriton niger, Concepción Pápalo, Thorius minutissimus, Craugastor pozo (panels, left to right) Back cover photograph: Collecting in Chamula, Chiapas Photo credits: The cover photographs were taken by the authors under grant agreements with the two main project funders: NGS and CEPF. -

Cortisol Regulation of Aquaglyceroporin HC-3 Protein Expression in the Erythrocytes of the Freeze Tolerant Tree Frog Dryophytes Chrysoscelis
University of Dayton eCommons Honors Theses University Honors Program 4-1-2019 Cortisol Regulation of Aquaglyceroporin HC-3 Protein Expression in the Erythrocytes of the Freeze Tolerant Tree Frog Dryophytes chrysoscelis Maria P. LaBello University of Dayton Follow this and additional works at: https://ecommons.udayton.edu/uhp_theses Part of the Biology Commons eCommons Citation LaBello, Maria P., "Cortisol Regulation of Aquaglyceroporin HC-3 Protein Expression in the Erythrocytes of the Freeze Tolerant Tree Frog Dryophytes chrysoscelis" (2019). Honors Theses. 218. https://ecommons.udayton.edu/uhp_theses/218 This Honors Thesis is brought to you for free and open access by the University Honors Program at eCommons. It has been accepted for inclusion in Honors Theses by an authorized administrator of eCommons. For more information, please contact [email protected], [email protected]. Cortisol Regulation of Aquaglyceroporin HC-3 Protein Expression in Erythrocytes from the Freeze Tolerant Tree Frog Dryophytes chrysoscelis Honors Thesis Maria P. LaBello Department: Biology Advisor: Carissa M. Krane, Ph.D. April 2019 Page | i Cortisol Regulation of Aquaglyceroporin HC-3 Protein Expression in the Erythrocytes of the Freeze Tolerant Tree Frog Dryophytes chrysoscelis Honors Thesis Maria P. LaBello Department: Biology Advisor: Carissa M. Krane, Ph.D. April 2019 Abstract Dryophytes chrysoscelis, commonly known as Cope’s gray treefrog, is a freeze tolerant anuran that freezes up to 65% of extracellular fluid during winter to survive. Glycerol is presumably used as a cryoprotectant during a period of cold-acclimation to protect cells from permanent damage due to hypoosmotic stress upon freezing and thawing. The passage of glycerol and water during cold-acclimation is mediated through aquaglyceroporin HC-3 in the nucleated erythrocytes (RBCs) of D. -

Multi-National Conservation of Alligator Lizards
MULTI-NATIONAL CONSERVATION OF ALLIGATOR LIZARDS: APPLIED SOCIOECOLOGICAL LESSONS FROM A FLAGSHIP GROUP by ADAM G. CLAUSE (Under the Direction of John Maerz) ABSTRACT The Anthropocene is defined by unprecedented human influence on the biosphere. Integrative conservation recognizes this inextricable coupling of human and natural systems, and mobilizes multiple epistemologies to seek equitable, enduring solutions to complex socioecological issues. Although a central motivation of global conservation practice is to protect at-risk species, such organisms may be the subject of competing social perspectives that can impede robust interventions. Furthermore, imperiled species are often chronically understudied, which prevents the immediate application of data-driven quantitative modeling approaches in conservation decision making. Instead, real-world management goals are regularly prioritized on the basis of expert opinion. Here, I explore how an organismal natural history perspective, when grounded in a critique of established human judgements, can help resolve socioecological conflicts and contextualize perceived threats related to threatened species conservation and policy development. To achieve this, I leverage a multi-national system anchored by a diverse, enigmatic, and often endangered New World clade: alligator lizards. Using a threat analysis and status assessment, I show that one recent petition to list a California alligator lizard, Elgaria panamintina, under the US Endangered Species Act often contradicts the best available science. -
For Review Only
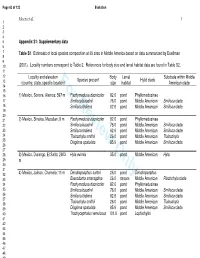
Page 63 of 123 Evolution Moen et al. 1 1 2 3 4 5 Appendix S1: Supplementary data 6 7 Table S1 . Estimates of local species composition at 39 sites in Middle America based on data summarized by Duellman 8 9 10 (2001). Locality numbers correspond to Table 2. References for body size and larval habitat data are found in Table S2. 11 12 Locality and elevation Body Larval Subclade within Middle Species present Hylid clade 13 (country, state, specific location)For Reviewsize Only habitat American clade 14 15 16 1) Mexico, Sonora, Alamos; 597 m Pachymedusa dacnicolor 82.6 pond Phyllomedusinae 17 Smilisca baudinii 76.0 pond Middle American Smilisca clade 18 Smilisca fodiens 62.6 pond Middle American Smilisca clade 19 20 21 2) Mexico, Sinaloa, Mazatlan; 9 m Pachymedusa dacnicolor 82.6 pond Phyllomedusinae 22 Smilisca baudinii 76.0 pond Middle American Smilisca clade 23 Smilisca fodiens 62.6 pond Middle American Smilisca clade 24 Tlalocohyla smithii 26.0 pond Middle American Tlalocohyla 25 Diaglena spatulata 85.9 pond Middle American Smilisca clade 26 27 28 3) Mexico, Durango, El Salto; 2603 Hyla eximia 35.0 pond Middle American Hyla 29 m 30 31 32 4) Mexico, Jalisco, Chamela; 11 m Dendropsophus sartori 26.0 pond Dendropsophus 33 Exerodonta smaragdina 26.0 stream Middle American Plectrohyla clade 34 Pachymedusa dacnicolor 82.6 pond Phyllomedusinae 35 Smilisca baudinii 76.0 pond Middle American Smilisca clade 36 Smilisca fodiens 62.6 pond Middle American Smilisca clade 37 38 Tlalocohyla smithii 26.0 pond Middle American Tlalocohyla 39 Diaglena spatulata 85.9 pond Middle American Smilisca clade 40 Trachycephalus venulosus 101.0 pond Lophiohylini 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 Evolution Page 64 of 123 Moen et al. -

Morphological Alterations in the External Gills of Some Tadpoles in Response to Ph
THIEME 142 Original Article Morphological Alterations in the External Gills of Some Tadpoles in Response to pH CaressaMaebhaThabah1 Longjam Merinda Devi1 Rupa Nylla Kynta Hooroo1 Sudip Dey2 1 Developmental Biology Laboratory, Department of Zoology, North- Address for correspondence Caressa Maebha Thabah, PhD in Eastern Hill University, Shillong, Meghalaya, India Zoology, Developmental Biology Laboratory, Department of Zoology, 2 Electron Microscope Laboratory, Sophisticated Analytical Instrument North-Eastern Hill University, Shillong 793022, Meghalaya, India Facility, North-Eastern Hill University, Shillong, Meghalaya, India (e-mail: [email protected]). J Morphol Sci 2018;35:142–152. Abstract Introduction Water pH affects the breeding, hatching, development, locomotion, mortality and habitat distributions of species in nature. The external gills of anuran tadpoles were studied by several authors in relation to abiotic factors. Exposure to low and high pH has been found to adversely affect the different tissues of various organisms. On that consideration, the present investigation was performed with tadpoles of the species Hyla annectans and Euphlyctis cyanophlyctis. Material and Methods The maximum and the minimum pH thresholds were determined prior to the detailed experiments on the effects of pH. The pH that demonstrated 50% mortality was taken as the minimum and maximum pH thresholds. The hatchlings of both the species were then subjected to different pH (based on the minimum and maximum pH thresholds). After 48 hours of exposure, the external gills of the hatchlings were anesthetized and observed under a scanning electron microscope. Keywords Results After 48 hours, clumping, overlapping and curling of the secondary filaments ► Hyla annectans of the external gills and epithelial lesions in response to both acidic and alkaline pH ► Euphlyctis were observed. -

Thermal Adaptation of Amphibians in Tropical Mountains
Thermal adaptation of amphibians in tropical mountains. Consequences of global warming Adaptaciones térmicas de anfibios en montañas tropicales: consecuencias del calentamiento global Adaptacions tèrmiques d'amfibis en muntanyes tropicals: conseqüències de l'escalfament global Pol Pintanel Costa ADVERTIMENT. La consulta d’aquesta tesi queda condicionada a l’acceptació de les següents condicions d'ús: La difusió d’aquesta tesi per mitjà del servei TDX (www.tdx.cat) i a través del Dipòsit Digital de la UB (diposit.ub.edu) ha estat autoritzada pels titulars dels drets de propietat intel·lectual únicament per a usos privats emmarcats en activitats d’investigació i docència. No s’autoritza la seva reproducció amb finalitats de lucre ni la seva difusió i posada a disposició des d’un lloc aliè al servei TDX ni al Dipòsit Digital de la UB. No s’autoritza la presentació del seu contingut en una finestra o marc aliè a TDX o al Dipòsit Digital de la UB (framing). Aquesta reserva de drets afecta tant al resum de presentació de la tesi com als seus continguts. En la utilització o cita de parts de la tesi és obligat indicar el nom de la persona autora. ADVERTENCIA. La consulta de esta tesis queda condicionada a la aceptación de las siguientes condiciones de uso: La difusión de esta tesis por medio del servicio TDR (www.tdx.cat) y a través del Repositorio Digital de la UB (diposit.ub.edu) ha sido autorizada por los titulares de los derechos de propiedad intelectual únicamente para usos privados enmarcados en actividades de investigación y docencia. -

Lista Patrón De Los Anfibios Y Reptiles De España (Actualizada a Diciembre De 2014)
See discussions, stats, and author profiles for this publication at: http://www.researchgate.net/publication/272447364 Lista patrón de los anfibios y reptiles de España (Actualizada a diciembre de 2014) TECHNICAL REPORT · DECEMBER 2014 CITATIONS READS 2 383 4 AUTHORS: Miguel A. Carretero Inigo Martinez-Solano University of Porto Estación Biológica de Doñana 256 PUBLICATIONS 1,764 CITATIONS 91 PUBLICATIONS 1,158 CITATIONS SEE PROFILE SEE PROFILE Enrique Ayllon Gustavo A. Llorente ASOCIACION HERPETOLOGICA ESPAÑOLA University of Barcelona 30 PUBLICATIONS 28 CITATIONS 210 PUBLICATIONS 1,239 CITATIONS SEE PROFILE SEE PROFILE Available from: Inigo Martinez-Solano Retrieved on: 13 November 2015 Lista patrón de los anfibios y reptiles de España | Diciembre 2014 Lista patrón de los anfibios y reptiles de España (Actualizada a diciembre de 2014) Editada por: Miguel A. Carretero Íñigo Martínez-Solano Enrique Ayllón Gustavo Llorente (Comisión permanente de taxonomía de la AHE) La siguiente lista patrón tiene como base la primera lista patrón: MONTORI, A.; LLORENTE, G. A.; ALONSO-ZARAZAGA, M. A.; ARRIBAS, O.; AYLLÓN, E.; BOSCH, J.; CARRANZA, S.; CARRETERO, M. A.; GALÁN, P.; GARCÍA-PARÍS, M.; HARRIS, D. J.; LLUCH, J.; MÁRQUEZ, R.; MATEO, J. A.; NAVARRO, P.; ORTIZ, M.; PÉREZ- MELLADO, V.; PLEGUEZUELOS, J. M.; ROCA, V.; SANTOS, X. & TEJEDO, M. (2005): Conclusiones de nomenclatura y taxonomía para las especies de anfibios y reptiles de España. MONTORI, A. & LLORENTE, G. A. (coord.). Asociación Herpetológica Española, Barcelona. En caso de aquellos ítems sin comentarios, la información correspondiente se encuentra en esta primera lista, que está descargable en: http://www.magrama.gob.es/es/biodiversidad/temas/inventarios- nacionales/lista_herpetofauna_2005_tcm7-22734.pdf Para aquellos ítems con nuevos comentarios, impliquen o no su modificación, se adjunta la correspondiente explicación con una clave numerada (#). -

Phylogeography of the American Green Treefrog Species Group
PHYLOGEOGRAPHY OF THE AMERICAN GREEN TREEFROG SPECIES GROUP by PAUL NATHANIEL PASICHNYK Presented to the Faculty of the Graduate School of The University of Texas at Arlington In Partial Fulfillment of the Requirements for the Degree of DOCTOR OF PHILOSOPHY THE UNIVERSITY OF TEXAS AT ARLINGTON November 2016 Copyright © by Paul N. Pasichnyk 2016 All Rights Reserved ii Acknowledgments A student’s graduate work is definitely not something that is done alone. I have received help from numerous people over the years and without their contributions, this would not have been possible. First, I would like to thank my parents for their tremendous support and motivation as I grew up and provided me with a foundation and love in education. Secondly, I would like to thank the person who has had to help me daily with tasks as simple as a pat on the back to as complex as reading a rough draft of a dissertation that might as well be in a foreign language, my wife Karen. My in-laws deserve special thanks as well, I do not believe that it is possible for others’ in-laws to be as supportive and helpful as mine. Then I would like to thank all the undergrads and friends who have helped see this to completion: Richard Hanna, Sari Mahon, Sarah Young, Nicole Lopez, Annamarie Slaven, Leslie Segovia, Pankaj BC, Matt Moseley, Christian Cox, Jeff Streicher, Claudia Marquez, Jesse Meik, Walter Schargel, Corey Roelke, Matt Watson, Rebbekah Watson, Thomas Eimermacher, Utpal Smart, David Sanchez, Jacobo Reyes-Velasco, Melissa Muenzler, Carl Franklin, Linda Taylor, Gloria Burlingham, Sherri Echols, and Paulette Williams. -

The Most Frog-Diverse Place in Middle America, with Notes on The
Offcial journal website: Amphibian & Reptile Conservation amphibian-reptile-conservation.org 13(2) [Special Section]: 304–322 (e215). The most frog-diverse place in Middle America, with notes on the conservation status of eight threatened species of amphibians 1,2,*José Andrés Salazar-Zúñiga, 1,2,3Wagner Chaves-Acuña, 2Gerardo Chaves, 1Alejandro Acuña, 1,2Juan Ignacio Abarca-Odio, 1,4Javier Lobon-Rovira, 1,2Edwin Gómez-Méndez, 1,2Ana Cecilia Gutiérrez-Vannucchi, and 2Federico Bolaños 1Veragua Foundation for Rainforest Research, Limón, COSTA RICA 2Escuela de Biología, Universidad de Costa Rica, San Pedro, 11501-2060 San José, COSTA RICA 3División Herpetología, Museo Argentino de Ciencias Naturales ‘‘Bernardino Rivadavia’’-CONICET, C1405DJR, Buenos Aires, ARGENTINA 4CIBIO Research Centre in Biodiversity and Genetic Resources, InBIO, Universidade do Porto, Campus Agrário de Vairão, Rua Padre Armando Quintas 7, 4485-661 Vairão, Vila do Conde, PORTUGAL Abstract.—Regarding amphibians, Costa Rica exhibits the greatest species richness per unit area in Middle America, with a total of 215 species reported to date. However, this number is likely an underestimate due to the presence of many unexplored areas that are diffcult to access. Between 2012 and 2017, a monitoring survey of amphibians was conducted in the Central Caribbean of Costa Rica, on the northern edge of the Matama mountains in the Talamanca mountain range, to study the distribution patterns and natural history of species across this region, particularly those considered as endangered by the International Union for Conservation of Nature. The results show the highest amphibian species richness among Middle America lowland evergreen forests, with a notable anuran representation of 64 species. -

<I>Rana Luteiventris</I>
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln USGS Staff -- Published Research US Geological Survey 2005 Population Structure of Columbia Spotted Frogs (Rana luteiventris) is Strongly Affected by the Landscape W. Chris Funk University of Texas at Austin, [email protected] Michael S. Blouin U.S. Geological Survey Paul Stephen Corn U.S. Geological Survey Bryce A. Maxell University of Montana - Missoula David S. Pilliod USDA Forest Service, [email protected] See next page for additional authors Follow this and additional works at: https://digitalcommons.unl.edu/usgsstaffpub Part of the Geology Commons, Oceanography and Atmospheric Sciences and Meteorology Commons, Other Earth Sciences Commons, and the Other Environmental Sciences Commons Funk, W. Chris; Blouin, Michael S.; Corn, Paul Stephen; Maxell, Bryce A.; Pilliod, David S.; Amish, Stephen; and Allendorf, Fred W., "Population Structure of Columbia Spotted Frogs (Rana luteiventris) is Strongly Affected by the Landscape" (2005). USGS Staff -- Published Research. 659. https://digitalcommons.unl.edu/usgsstaffpub/659 This Article is brought to you for free and open access by the US Geological Survey at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in USGS Staff -- Published Research by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Authors W. Chris Funk, Michael S. Blouin, Paul Stephen Corn, Bryce A. Maxell, David S. Pilliod, Stephen Amish, and Fred W. Allendorf This article is available at DigitalCommons@University of Nebraska - Lincoln: https://digitalcommons.unl.edu/ usgsstaffpub/659 Molecular Ecology (2005) 14, 483–496 doi: 10.1111/j.1365-294X.2005.02426.x BlackwellPopulation Publishing, Ltd. structure of Columbia spotted frogs (Rana luteiventris) is strongly affected by the landscape W.