Introduction and General Organization of the Nervous System Course
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

NS201C Anatomy 1: Sensory and Motor Systems
NS201C Anatomy 1: Sensory and Motor Systems 25th January 2017 Peter Ohara Department of Anatomy [email protected] The Subdivisions and Components of the Central Nervous System Axes and Anatomical Planes of Sections of the Human and Rat Brain Development of the neural tube 1 Dorsal and ventral cell groups Dermatomes and myotomes Neural crest derivatives: 1 Neural crest derivatives: 2 Development of the neural tube 2 Timing of development of the neural tube and its derivatives Timing of development of the neural tube and its derivatives Gestational Crown-rump Structure(s) age (Weeks) length (mm) 3 3 cerebral vesicles 4 4 Optic cup, otic placode (future internal ear) 5 6 cerebral vesicles, cranial nerve nuclei 6 12 Cranial and cervical flexures, rhombic lips (future cerebellum) 7 17 Thalamus, hypothalamus, internal capsule, basal ganglia Hippocampus, fornix, olfactory bulb, longitudinal fissure that 8 30 separates the hemispheres 10 53 First callosal fibers cross the midline, early cerebellum 12 80 Major expansion of the cerebral cortex 16 134 Olfactory connections established 20 185 Gyral and sulcul patterns of the cerebral cortex established Clinical case A 68 year old woman with hypertension and diabetes develops abrupt onset numbness and tingling on the right half of the face and head and the entire right hemitrunk, right arm and right leg. She does not experience any weakness or incoordination. Physical Examination: Vitals: T 37.0° C; BP 168/87; P 86; RR 16 Cardiovascular, pulmonary, and abdominal exam are within normal limits. Neurological Examination: Mental Status: Alert and oriented x 3, 3/3 recall in 3 minutes, language fluent. -

Somatosensory Systems
Somatosensory Systems Sue Keirstead, Ph.D. Assistant Professor Dept. of Integrative Biology and Physiology Stem Cell Institute E-mail: [email protected] Tel: 612 626 2290 Class 9: Somatosensory System (p. 292-306) 1. Describe the 3 main types of somatic sensations: 1. tactile: light touch, deep pressure, vibration, cold, hot, etc., 2. pain, 3. Proprioception. 2. List the types of sensory receptors that are found in the skin (Figure 9.11) and explain what determines the optimum type of stimulus that will activate each. 3. Describe the two different modality-specific ascending somatosensory pathways and note which modalities are carried in each (Figure 9.10 and 9.13). 4. Describe how it is possible for us to differentiate between stimuli of different modalities in the same body part (i.e. fingertip). Consider this at the level of 1) the sensory receptors and 2) the neurons onto which they synapse in the ascending sensory systems. 5. Explain how one might determine the location of a spinal cord injury based on the modality of sensation that is lost and the region of the body (both the side of the body and body part) where sensation is lost (Figure 9.18). 6. Describe how incoming sensory inputs from primary sensory axons can be modified at the level of the spinal cord and relate this to the mechanism of action of some common pain medications (Figure 9-18). 7. Describe the homunculus and explain the significance of the size of the region of the somatosensory cortex devoted to a particular body part. Cerebral cortex Interneuron Thalamus Interneuron 4 Integration of sensory Stimulus input in the CNS 1 Stimulation Sensory Axon of sensory of sensory receptor neuron receptor Graded potential Action potentials 2 Transduction 3 Generation of of the stimulus action potentials Copyright © 2016 by John Wiley & Sons, Inc. -

Nerve Ultrasound in Dorsal Root Ganglion Disorders: Smaller Nerves Lead to Bigger Insights
Clinical Neurophysiology 130 (2019) 550–551 Contents lists available at ScienceDirect Clinical Neurophysiology journal homepage: www.elsevier.com/locate/clinph Editorial Nerve ultrasound in dorsal root ganglion disorders: Smaller nerves lead to bigger insights See Article, pages 568–572 After decades of having to make do with electric stimulation representing the fascicles, bundled together in a large outer cable and recording (i.e. nerve conduction studies, electromyography sheath (van Alfen et al., 2018). and evoked potentials), nerve ultrasound now provides the oppor- Next, it is important to realize what the ratio between axon/ tunity to improve neurodiagnostic patient care by deploying a myelin and connective tissue in a given nerve segment is, and powerful tool to detect neuromuscular pathology in an accurate how that ratio changes from the proximal root to the distal end and patient-friendly way (Mah et al., 2018; Walker et al., 2018). branches (Schraut et al., 2016). Connective tissue elements of the Nerve ultrasound is also increasingly providing neurologists and perineurium and epineurium are relatively sparse at the very prox- clinical neurophysiologists with the opportunity to increase their imal root and plexus levels, with an average connective tissue con- insight in the pathophysiology of peripheral nervous system tent of around 25–30%. Ultrasonographically, this means that roots (PNS) pathology. In this issue of Clinical Neurophysiology, Leadbet- will always look rather black in appearance without much dis- ter and coworkers (Leadbetter et al., 2019) describe the results of cernible fascicular architecture, as the sparseness of connective tis- their study on nerve ultrasound for diagnosing sensory neuronopa- sue elements provides relatively few reflectors to create an image thy in spinocerebellar ataxia type 2 and CANVAS syndrome. -

Thalamus and Limbic System
Prof. Saeed Abuel Makarem 1 Objectives By the end of the lecture, you should be able to: Describe the anatomy and main functions of the thalamus. Name and identify different nuclei of the thalamus. Describe the main connections and functions of thalamic nuclei. Name and identify different parts of the limbic system. Describe main functions of the limbic system. Describe the effects of lesions of the limbic system. It is the largest nuclear mass of Thalamus the whole body. It is the largest part of the THALAMUS diencephalon It is formed of two oval masses Corpus callosum of grey matter. It is the gateway to the Midbrain cortex. Resemble a PONS small hen. Together with the hypothalamus they form the lateral wall of the 3rd ventricle. 3 It sends received Thalamus information to the cerebral cortex from different brain regions. Axons from every sensory system (except olfaction) synapse in the thalamus as the last relay site 'last pit stop' before the information reaches the cerebral cortex. There are some thalamic nuclei that receive input from: 1. Cerebellar nuclei, 2. Basal ganglia- and 3. Limbic-related brain regions. 4 It has 4 surfaces & 2 ends. Relations Surfaces Lateral:(L) Posterior limb of the internal capsule. Medial: (3) The 3rd ventricle. In some people the 2 thalami are connected to ach other by interthalamic adhesion S (connexus,) or Massa intermedia, which crosses L through the 3rd ventricle. 3 Superior: (s) I Lateral ventricle and fornix. Inferior: Hypothalamus, anteriorly & Subthalamus posteriorly. 5 Anterior end: Forms a projection, called the anterior tubercle. It lies just behind the interventricular foramen. -

Proprioceptive- Deep Tendon Reflex Dr. Jose Palomar
PROPRIOCEPTIVE- DEEP TENDON REFLEX DR. JOSE PALOMAR PROPRIOCEPTIVE- DEEP TENDON REFLEX DR. JOSE PALOMAR Proprioceptive-Deep Tendon Reflex About the Author Dr. Jose Palomar Lever, M.D. is a native of Guadalajara, the capital city of the state of Jalisco in Mexico. He began his medical school education at the age of 17 at the Universidad Autónoma de Guadalajara (UAG) and received his training in Orthopedic Surgery and Traumatology at the Universidad del Ejercito y Fuerza Aérea (UDEFA). He performed his first orthopedic surgery at the age of 24 and between 1984 and 1988 was an orthopedic surgeon on the staff of the Reconstructive and Plastic Surgery Institute of Jalisco, S.S.A. He went on to receive specialized training in minimally invasive spine surgery at the Texas Back Institute in Dallas, Texas. Pursuing his interest in what he now refers to as the “software” of the human body, a study, which began in earnest for him in 2000, Dr. Palomar became a Diplomate in Applied Kinesiology from the International College of Applied Kinesiology (ICAK). He received the organization's Alan Beardall Memorial Award for Research for 2004-2005 and over the years has had eighteen papers accepted for inclusion in ICAK-USA Proceedings. He also completed the Carrick Institute for Graduate Studies program in Clinical Neurology. Today, in addition to pursuing an ongoing research program, Dr. Palomar conducts regular trainings in Proprioceptive-Deep Tendon Reflex (P-DTR) for medical practitioners in USA, Canada, Australia, Mexico, England, Poland, Latvia and Russia, and continues to practice medicine from his home base in Guadalajara, Mexico. -

Anatomy-Nerve Tracking
INJECTABLES ANATOMY www.aestheticmed.co.uk Nerve tracking Dr Sotirios Foutsizoglou on the anatomy of the facial nerve he anatomy of the human face has received enormous attention during the last few years, as a plethora of anti- ageing procedures, both surgical and non-surgical, are being performed with increasing frequency. The success of each of those procedures is greatly dependent on Tthe sound knowledge of the underlying facial anatomy and the understanding of the age-related changes occurring in the facial skeleton, ligaments, muscles, facial fat compartments, and skin. The facial nerve is the most important motor nerve of the face as it is the sole motor supply to all the muscles of facial expression and other muscles derived from the mesenchyme in the embryonic second pharyngeal arch.1 The danger zone for facial nerve injury has been well described. Confidence when approaching the nerve and its branches comes from an understanding of its three dimensional course relative to the layered facial soft tissue and being aware of surface anatomy landmarks and measurements as will be discussed in this article. Aesthetic medicine is not static, it is ever evolving and new exciting knowledge emerges every day unmasking the relationship of the ageing process and the macroscopic and microscopic (intrinsic) age-related changes. Sound anatomical knowledge, taking into consideration the natural balance between the different facial structures and facial layers, is fundamental to understanding these changes which will subsequently help us develop more effective, natural, long-standing and most importantly, safer rejuvenating treatments and procedures. The soft tissue of the face is arranged in five layers: 1) Skin; 2) Subcutaneous fat layer; 3) Superficial musculoaponeurotic system (SMAS); 4) Areolar tissue or loose connective tissue (most clearly seen in the scalp and forehead); 5) Deep fascia formed by the periosteum of facial bones and the fascial covering of the muscles of mastication (lateral face). -

Meninges,Cerebrospinal Fluid, and the Spinal Cord
The Nervous System SPINAL CORD Spinal Cord Continuation of CNS inferior to foramen magnum (medulla) Simpler Conducts impulses to and from brain Two way conduction pathway Reflex actions Spinal Cord Passes through vertebral canal Foramen magnum L2 Conus medullaris Filum terminale Cauda equina Cervical Cervical spinal nerves enlargement Dura and arachnoid Thoracic mater spinal nerves Lumbar enlargement Conus medullaris Lumbar Cauda spinal nerves equina Filum (a) The spinal cord and its nerve terminale Sacral roots, with the bony vertebral spinal nerves arches removed. The dura mater and arachnoid mater are cut open and reflected laterally. Figure 12.29a Spinal Cord Spinal nerves 31 pairs Cervical and lumbar enlargements The nerves serving the upper and lower limbs emerge here Cervical Cervical spinal nerves enlargement Dura and arachnoid Thoracic mater spinal nerves Lumbar enlargement Conus medullaris Lumbar Cauda spinal nerves equina Filum (a) The spinal cord and its nerve terminale Sacral roots, with the bony vertebral spinal nerves arches removed. The dura mater and arachnoid mater are cut open and reflected laterally. Figure 12.29a Spinal Cord Protection Bone, meninges, and CSF Spinal tap-inferior to second lumbar vertebra T12 Ligamentum flavum L5 Lumbar puncture needle entering subarachnoid space L4 Supra- spinous ligament L5 Filum terminale S1 Inter- Cauda equina vertebral Arachnoid Dura in subarachnoid disc matter mater space Figure 12.30 Spinal Cord Cross section Central gray matter Cortex of white matter Epidural -

Spinal Nerves, Ganglia, and Nerve Plexus Spinal Nerves
Chapter 13 Spinal Nerves, Ganglia, and Nerve Plexus Spinal Nerves Posterior Spinous process of vertebra Posterior root Deep muscles of back Posterior ramus Spinal cord Transverse process of vertebra Posterior root ganglion Spinal nerve Anterior ramus Meningeal branch Communicating rami Anterior root Vertebral body Sympathetic ganglion Anterior General Anatomy of Nerves and Ganglia • Spinal cord communicates with the rest of the body by way of spinal nerves • nerve = a cordlike organ composed of numerous nerve fibers (axons) bound together by connective tissue – mixed nerves contain both afferent (sensory) and efferent (motor) fibers – composed of thousands of fibers carrying currents in opposite directions Anatomy of a Nerve Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Epineurium Perineurium Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Endoneurium Nerve Rootlets fiber Posterior root Fascicle Posterior root ganglion Anterior Blood root vessels Spinal nerve (b) Copyright by R.G. Kessel and R.H. Kardon, Tissues and Organs: A Text-Atlas of Scanning Electron Microscopy, 1979, W.H. Freeman, All rights reserved Blood vessels Fascicle Epineurium Perineurium Unmyelinated nerve fibers Myelinated nerve fibers (a) Endoneurium Myelin General Anatomy of Nerves and Ganglia • nerves of peripheral nervous system are ensheathed in Schwann cells – forms neurilemma and often a myelin sheath around the axon – external to neurilemma, each fiber is surrounded by -

Tissue Engineered Myelination and the Stretch Reflex Arc Sensory Circuit: Defined Medium Ormulation,F Interface Design and Microfabrication
University of Central Florida STARS Electronic Theses and Dissertations, 2004-2019 2009 Tissue Engineered Myelination And The Stretch Reflex Arc Sensory Circuit: Defined Medium ormulation,F Interface Design And Microfabrication John Rumsey University of Central Florida Part of the Biology Commons Find similar works at: https://stars.library.ucf.edu/etd University of Central Florida Libraries http://library.ucf.edu This Doctoral Dissertation (Open Access) is brought to you for free and open access by STARS. It has been accepted for inclusion in Electronic Theses and Dissertations, 2004-2019 by an authorized administrator of STARS. For more information, please contact [email protected]. STARS Citation Rumsey, John, "Tissue Engineered Myelination And The Stretch Reflex Arc Sensory Circuit: Defined Medium Formulation, Interface Design And Microfabrication" (2009). Electronic Theses and Dissertations, 2004-2019. 3826. https://stars.library.ucf.edu/etd/3826 TISSUE ENGINEERED MYELINATION AND THE STRETCH REFLEX ARC SENSORY CIRCUIT: DEFINED MEDIUM FORMULATION, INTERFACE DESIGN AND MICROFABRICATION by JOHN WAYNE RUMSEY B.S. University of Florida, 2001 M.S. University of Central Florida, 2004 A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Burnett School of Biomedical Sciences in the College of Medicine at the University of Central Florida Orlando, Florida Fall Term 2009 Major Professor: James J. Hickman ABSTRACT The overall focus of this research project was to develop an in vitro tissue- engineered system that accurately reproduced the physiology of the sensory elements of the stretch reflex arc as well as engineer the myelination of neurons in the systems. In order to achieve this goal we hypothesized that myelinating culture systems, intrafusal muscle fibers and the sensory circuit of the stretch reflex arc could be bioengineered using serum-free medium formulations, growth substrate interface design and microfabrication technology. -

The Peripheral Nervous System
The Peripheral Nervous System Dr. Ali Ebneshahidi Peripheral Nervous System (PNS) – Consists of 12 pairs of cranial nerves and 31 pairs of spinal nerves. – Serves as a critical link between the body and the central nervous system. – peripheral nerves contain an outermost layer of fibrous connective tissue called epineurium which surrounds a thinner layer of fibrous connective tissue called perineurium (surrounds the bundles of nerve or fascicles). Individual nerve fibers within the nerve are surrounded by loose connective tissue called endoneurium. Cranial Nerves Cranial nerves are direct extensions of the brain. Only Nerve I (olfactory) originates from the cerebrum, the remaining 11 pairs originate from the brain stem. Nerve I (Olfactory)- for the sense of smell (sensory). Nerve II (Optic)- for the sense of vision (sensory). Nerve III (Oculomotor)- for controlling muscles and accessory structures of the eyes ( primarily motor). Nerve IV (Trochlear)- for controlling muscles of the eyes (primarily motor). Nerve V (Trigeminal)- for controlling muscles of the eyes, upper and lower jaws and tear glands (mixed). Nerve VI (Abducens)- for controlling muscles that move the eye (primarily motor). Nerve VII (Facial) – for the sense of taste and controlling facial muscles, tear glands and salivary glands (mixed). Nerve VIII (Vestibulocochlear)- for the senses of hearing and equilibrium (sensory). Nerve IX (Glossopharyngeal)- for controlling muscles in the pharynx and to control salivary glands (mixed). Nerve X (Vagus)- for controlling muscles used in speech, swallowing, and the digestive tract, and controls cardiac and smooth muscles (mixed). Nerve XI (Accessory)- for controlling muscles of soft palate, pharynx and larynx (primarily motor). Nerve XII (Hypoglossal) for controlling muscles that move the tongue ( primarily motor). -
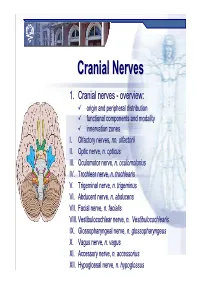
Cranial Nervesnerves
CranialCranial NervesNerves 1. Cranial nerves - overview: origin and peripheral distribution functional components and modality innervation zones I. Olfactory nerves, nn. olfactorii II. Optic nerve, n. opticus III. Oculomotor nerve, n. oculomotorius IV. Trochlear nerve, n. trochlearis V. Trigeminal nerve, n. trigeminus VI. Abducent nerve, n. abducens VII. Facial nerve, n. facialis VIII. Vestibulocochlear nerve, n. Vestibulocochlearis IX. Glossopharyngeal nerve, n. glossopharyngeus X. Vagus nerve, n. vagus XI. Accessory nerve, n. accessorius XII. Hypoglossal nerve, n. hypoglossus Cranial nerves CranialCranial nervesnerves 0. N. terminalis I. N. olfactorius II. N. opticus III. N. oculomotorius IV. N. trochlearis V. N. trigeminus VI. N. abducens VII. N. facialis VIII. N. vestibulocochlearis IX. N. glossopharyngeus X. N. vagus XI. N. accessorius XII. N. hypoglossus Prof. Dr. Nikolai Lazarov 2 Cranial nerves FunctionalFunctional classificationclassification purely sensory (afferent ): n. olfactorius n. opticus n. vestibulocochlearis purely motor (efferent): n. oculomotorius n. trochlearis n. abducens n. accessorius n. hypoglossus mixed (sensory&motor ): n. trigeminus n. facialis n. glossopharyngeus n. vagus autonomic (parasympathetic( ): n. oculomotorius n. facialis n. glossopharyngeus n. vagus Prof. Dr. Nikolai Lazarov 3 Cranial nerves AnatomicAnatomic relationshipsrelationships Location within the brainstem: ventrally: n. olfactorius n. opticus n. oculomotorius n. abducens n. hypoglossus laterally: n. trigeminus -

BRS Physiology 3Rd Edition
Board Review Series • Reflects USMLE changes • Approximately 350 USMLE-type questions with explanations • Numerous illustrations, diagrams, and tables • Easy-to-follow outline covering all USMLE-tested topics • A comprehensive examination V Ah, LIPPINCOTT -"*" WILLIAMS &WILKIN; mum IEEE mows IF 'IMP IMMO MINK I I. Key Physiology Topics for USMLE Step I Cell Physiology Transport mechanisms Ionic basis for action potential Excitation-contraction coupling in skeletal, cardiac, and smooth muscle Neuromuscular transmission Autonomic Physiology Cholinergic receptors Adrenergic receptors Effects of autonomic nervous system on organ system function Cardiovascular Physiology Events of cardiac cycle Pressure, flow, resistance relationships Frank-Starling law of the heart Ventricular pressure-volume loops Ionic basis for cardiac action potentials Starling forces in capillaries Regulation of arterial pressure (baroreceptors and renin-angiotensin II-aldosterone system) Cardiovascular and pulmonary responses to exercise Cardiovascular responses to hemorrhage Cardiovascular responses to changes in posture Respiratory Physiology Lung and chest-wall compliance curves Breathing cycle Hemoglobin-02 dissociation curve Causes of hypoxemia and hypoxia vq, P02, and P00 2 in upright lung V/Q defects Peripheral and central chemoreceptors in control of breathing Responses to high altitude Renal and Acid-Base Physiology Fluid shifts between body fluid compartments Starling forces across glomerular capillaries Transporters in various segments of nephron (Na Cl-,