Microbial Responses to Different Operating Practices for Biogas Production Systems Maria Westerholm and Anna Schnürer
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download/Issues/Mining/Reference Guide to Treatment Technologi Es for MIW.Pdf
Removal of Soluble Selenium in the Presence of Nitrate from Coal Mining-Influenced Water by Frank Nkansah - Boadu BSc., Kwame Nkrumah University of Science and Technology, 2003 MASc., The University of British Columbia, 2013 A DISSERTATION SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY in THE FACULTY OF GRADUATE AND POSTDOCTORAL STUDIES (Chemical and Biological Engineering) THE UNIVERSITY OF BRITISH COLUMBIA (Vancouver) December 2019 © Frank Nkansah - Boadu, 2019 The following individuals certify that they have read, and recommend to the Faculty of Graduate and Postdoctoral Studies for acceptance, the dissertation entitled: Removal of Soluble Selenium in the Presence of Nitrate from Coal Mining-Influenced Water submitted by Frank Nkansah-Boadu in partial fulfillment of the requirements for the degree of Doctor of Philosophy In Chemical and Biological Engineering Examining Committee: Susan Baldwin, Chemical and Biological Engineering Supervisor Vikramaditya Yadav, Chemical and Biological Engineering Supervisory Committee Member Troy Vassos, Adjunct Professor, Civil Engineering Supervisory Committee Member Anthony Lau, Chemical and Biological Engineering University Examiner Scott Dunbar, Mining Engineering University Examiner ii Abstract Biological treatment to remove dissolved selenium from mining-influenced water (MIW) is inhibited by co-contaminants, especially nitrate. It was hypothesized that selenium reducing microorganisms can be obtained from native mine bacteria at sites affected by MIW due to the selection pressure from elevated selenium concentrations at those sites. Enrichment of these microorganisms and testing of their capacity to remove dissolved selenium from actual coal MIW was the objective of this dissertation. Fifteen sediments were collected from eleven different vegetated or non-vegetated seepage collection ponds and one non-impacted natural wetland. -

Revised Supplement 1: Reference List for Figure 1
Revised Supplement 1: Reference list for Figure 1. Manuscript title: Process disturbances in agricultural biogas production – causes, mechanisms and effects on the biogas microbiome: A review Susanne Theuerl 1,*, Johanna Klang 1, Annette Prochnow 1,2 1 Leibniz Institute for Agricultural Engineering and Bioeconomy, Max-Exth-Allee 100, 14469 Potsdam, Germany, [email protected] (ST), [email protected] (JK), [email protected] (AP) 2 Humboldt-Universität zu Berlin, Albrecht-Daniel-Thaer-Institute for Agricultural and Horticultural Sciences, Hinter der Reinhardtstr. 6-8, 10115 Berlin, Germany * Correspondence: [email protected] Tel.: +49-331-5699-900 References of Figure 1 1Abt et al. 2010, 2Parizzi et al. 2012, 3Hahnke et al. 2016 and Tomazetto et al. 2018, 4Ueki et al. 2006 and Gronow et al. 2011, 5Grabowski et al. 2005, 6Chen and Dong 2005, 7Avgustin et al. 1997 and Purushe et al. 2010, 8Yamada et al. 2006 and Matsuura et al. 2015, 9Yamada et al. 2007 and Matsuura et al. 2015, 10Sun et al. 2016, 11Suen et al. 2011, 12Hahnke et al. 2014 and Tomazetto et al. 2016, 13Mechichi et al. 1999, 14Koeck et al. 2015a and 2015b, 15Tomazetto et al. 2017, 16Fonknechten et al. 2010, 17Chen et al. 2010, 18Nishiyama et al. 2009, 19Sieber et al. 2010, 20Plerce et al. 2008, 21Westerholm et al. 2011 and Müller et al. 2015, 22Ueki et al. 2014, 23Jackson et al. 1999 and McInerney et al. 2007, 24Ma et al. 2017, 25Harmsen et al. 1998 and Plugge et al. 2012, 26Menes and Muxi 2002, Mavromatis et al. 2013 and Hania et al. -

Lascolabacillus Massiliensis'': a New Species Isolated
NEW MICROBES IN HUMANS “Lascolabacillus massiliensis”: a new consent, and the agreement of the National Ethics Committee of Senegal and the local ethics committee of the IFR48 species isolated from the human gut (Marseille, France) were obtained under numbers 11-017 and 09-022, respectively. The initial growth was obtained after 10 days of culture in a 5% sheep blood-enriched sheep rumen M. Beye, S. Bakour, S. I. Traore, D. Raoult and P.-E. Fournier medium in aerobic atmosphere at 37°C. The bacterium was Unité de Recherche en Maladies Infectieuses et Tropicales Emergentes, sub-cultured on 5% sheep blood-enriched Columbia agar Institut Hospitalo-Universitaire Méditerranée-Infection, Aix-Marseille (bioMérieux, Marcy l’Etoile, France) and grew in 24 hours at Université, Faculté de Médecine, Marseille cedex 5, France 37°C in both aerobic and anaerobic conditions. Agar-grown colonies were pale grey and 1.5 mm in diameter. Bacterial cells were Gram-negative, rod-shaped and polymorphic, ranginginlengthfrom1.5to10μm. Strain SIT8 was catalase- Abstract and oxidase-negative. The 16S rRNA gene was sequenced using the fD1-rP2 primers as previously described, using a 3130-XL sequencer (Applied Biosciences, Saint Aubin, We report here the main characteristics of “Lascolabacillus France). Strain SIT8 exhibited a 94.14% sequence identity with massiliensis” strain SIT8 (CSUR P1560) that was isolated from the Proteiniphilum acetatigenes strain TB107 (GenBank accession stool of a healthy 28-month-old boy. NR_043154), the phylogenetically closest species with New Microbes and New Infections © 2016 The Authors. Published standing in nomenclature (Fig. 1), which putatively classifies it by Elsevier Ltd on behalf of European Society of Clinical as a member of a new genus within the family Porphyr- Microbiology and Infectious Diseases. -
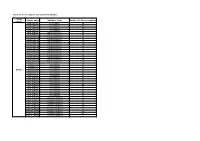
Table S1: List of Samples Included in the Analysis
Table S1: list of samples included in the analysis Study Sample name Inhibitory status Number of days at sampling number DNA.0P2T4 No inhibition 29 DNA.0P2T6 No inhibition 57 DNA.10P2T4 No inhibition 29 DNA.10P2T6 No inhibition 57 DNA.75P2T4 Phenol inhibition 29 DNA.75P2T6 Phenol inhibition 57 DNA.100P2T4 Phenol inhibition 29 DNA.100P2T6 Phenol inhibition 57 DNA.125P1T4 Phenol inhibition 29 DNA.125P1T6 Phenol inhibition 57 DNA.125P2T4 Phenol inhibition 29 DNA.125P2T6 Phenol inhibition 57 DNA.125P3T4 Phenol inhibition 29 DNA.125P3T6 Phenol inhibition 57 DNA.150P2T4 Phenol inhibition 29 DNA.150P2T6 Phenol inhibition 57 DNA.200P2T4 Phenol inhibition 29 DNA.200P2T6 Phenol inhibition 57 DNA.0N2T4 No inhibition 29 DNA.0N2T5 No inhibition 42 DNA.0N2T6 No inhibition 57 Study 1 DNA.5N2T4 No inhibition 29 DNA.5N2T5 No inhibition 42 DNA.5N2T6 No inhibition 57 DNA.10N2T4 No inhibition 29 DNA.10N2T5 No inhibition 42 DNA.10N2T6 No inhibition 57 DNA.15N2T4 No inhibition 29 DNA.15N2T5 No inhibition 42 DNA.15N2T6 No inhibition 57 DNA.25N2T4 No inhibition 29 DNA.25N2T5 No inhibition 42 DNA.25N2T6 No inhibition 57 DNA.75N2T4 Ammonia inhibition 29 DNA.75N2T5 Ammonia inhibition 42 DNA.75N2T6 Ammonia inhibition 57 DNA.100N2T4 Ammonia inhibition 29 DNA.100N2T5 Ammonia inhibition 42 DNA.100N2T6 Ammonia inhibition 57 DNA.250N2T4 Ammonia inhibition 29 DNA.250N2T5 Ammonia inhibition 42 DNA.250N2T6 Ammonia inhibition 57 nono2T3 No inhibition 16 noN2T4 Ammonia inhibition 23 noN2T8 Ammonia inhibition 60 noN2T9 Ammonia inhibition 85 noPhi2T4 Phenol inhibition 23 noPhi2T5 -

Thermanaerovibrio Acidaminovorans Gen
[nternationd Journal ofSystematic Bacterio/ogy (1999), 49,969-974 Printed in Great Britain Phylogenetic relationships of three amino-acid-utilizing anaerobes, Selenomonas acidaminovorans, 'Selenomonas acidaminophila ' and Eubacterium acidaminophilum, as inferred from partial 16s rDNA nucleotide sequences and proposal of Thermanaerovibrio acidaminovorans gen. nov., comb. nov. and Anaeromusa acidaminophila gen. nov., comb. nov. H. Sandra Baena,lr2 Marie-LaureFardeau,' T. S. WOO,^ Bernard Ollivier,l Marc Labat' and Bharat K. C. Pate13 Author for correspondence: Bharat K. C. Patel. Tel: +61 417 726 671. Fax: +61 7 3875 7656. e-mail : [email protected] 1 Laboratoire ORSTOM de 16s rRNA gene sequences of three previously described amino-acid-fermenting Microbiologie des anaerobes, Selenomonas acidaminovorans, 'Selenomonas acidaminophila and AnaBrobies, Universite de Provence, CESB-ESILCase Eubacterium acidaminophilum, were determined. All three were found to 925,163 Avenue de cluster within the Clostridium and related genera of the subphylum of the Luminy, 13288 Marseille Gram-positive bacteria. The thermophile, S. acidaminovorans, formed ap Cedex 9, France individual line of descent and was equidistantly placed between 2 Departamento de Biologia, Dethiosulfovibriopeptidovorans and Anaerobaculum íhermoterrenum Pontificia Universidad Javeriana, PO6 56710, (similarity of 85%), both of which also form single lines of descent. '5. SantaFe de Bogota, acidaminophila ' was related to Clostridium quercicolum, a member of cluster Colombia IX, with a similarity of 90%, whereas E. acidaminophilum was closely related School of Biomolecular to Clostridium litorale (similarity of 96%) as a member of cluster XI. Based on and Biomedical Sciences, the phylogenetic data presented in this report and the phenotypic descriptions Faculty of Science, Griffith University, Brisbane, of these bacteria published previously, it is recommended that S. -

Membrane Fouling and Visualisation Studies
The Potential of Water Hyacinth (Eicchornia crassipes) from Hartbeespoort dam in Biogas and Soil Ameliorant production, as a Solution to Water Weed Challenges Report to the WATER RESEARCH COMMISSION by A Roopnarain, R Adeleke, R Makofane, L Obi, M Lubinga Agricultural Research Council WRC Report No. 2543/1/18 ISBN 978-0-6392-0024-8 September 2018 Obtainable from Water Research Commission Private Bag X03 GEZINA, 0031 [email protected] or download from www.wrc.org.za DISCLAIMER This report has been reviewed by the Water Research Commission (WRC) and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the WRC nor does mention of trade names or commercial products constitute endorsement or recommendation for use. Printed in the Republic of South Africa © Water Research Commission ii iii iv EXECUTIVE SUMMARY BACKGROUND The Hartbeespoort Dam is situated in the North West province of South Africa and is one of the most significant dams in the economic hub of the province and the Crocodile (West) Marico (CWM) Water Management Area (WMA). The dam is primarily utilised for domestic, industrial, agricultural and recreational purposes. The numerous ecosystem services provided by the dam contribute to its economic significance. These services include provisioning (the availability of water for abstraction), supporting services (holiday, commercial and residential) and regulatory (waste assimilation). City dwellers are frequently attracted to the large water body that is situated within a mountainous setting. This has contributed to the increasing importance of the dam as a regional tourist and recreational centre. The socio-economic activities and facilities available at the dam include holiday resorts, conference venues, weekend cottages, golf courses, fishing, boating and water-skiing. -

Supplementary Figure Legends for Rands Et Al. 2019
Supplementary Figure legends for Rands et al. 2019 Figure S1: Display of all 485 prophage genome maps predicted from Gram-Negative Firmicutes. Each horizontal line corresponds to an individual prophage shown to scale and color-coded for annotated phage genes according to the key displayed in the right- side Box. The left vertical Bar indicates the Bacterial host in a colour code. Figure S2: Projection of virome sequences from 183 human stool samples on A. Acidaminococcus intestini RYC-MR95, and B. Veillonella parvula UTDB1-3. The first panel shows the read coverage (Y-axis) across the complete Bacterial genome sequence (X-axis; with bp coordinates). Predicted prophage regions are marked with red triangles and magnified in the suBsequent panels. Virome reads projected outside of prophage prediction are listed in Table S4. Figure S3: The same display of virome sequences projected onto Bacterial genomes as in Figure S2, But for two different Negativicute species: A. Dialister Marseille, and B. Negativicoccus massiliensis. For non-phage peak annotations, see Table S4. Figure S4: Gene flanking analysis for the lysis module from all prophages predicted in all the different Bacterial clades (Table S2), a total of 3,462 prophages. The lysis module is generally located next to the tail module in Firmicute prophages, But adjacent to the packaging (terminase) module in Escherichia phages. 1 Figure S5: Candidate Mu-like prophage in the Negativicute Propionispora vibrioides. Phage-related genes (arrows indicate transcription direction) are coloured and show characteristics of Mu-like genome organization. Figure S6: The genome maps of Negativicute prophages harbouring candidate antiBiotic resistance genes MBL (top three Veillonella prophages) and tet(32) (bottom Selenomonas prophage remnant); excludes the ACI-1 prophage harbouring example characterised previously (Rands et al., 2018). -

WO 2014/135633 Al 12 September 2014 (12.09.2014) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization I International Bureau (10) International Publication Number (43) International Publication Date WO 2014/135633 Al 12 September 2014 (12.09.2014) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every C12N 9/04 (2006.01) C12P 7/16 (2006.01) kind of national protection available): AE, AG, AL, AM, C12N 9/88 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, (21) Number: International Application DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, PCT/EP2014/054334 HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, (22) International Filing Date: KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, 6 March 2014 (06.03.2014) MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, (25) Filing Language: English SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, (26) Publication Language: English TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: 13 158012.8 6 March 2013 (06.03.2013) EP (84) Designated States (unless otherwise indicated, for every kind of regional protection available): ARIPO (BW, GH, (71) Applicants: CLARIANT PRODUKTE (DEUTSCH- GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, LAND) GMBH [DE/DE]; Briiningstrasse 50, 65929 UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, Frankfurt am Main (DE). -

Characterization of Ruminal Bacteria in Grazing Nellore Steers
248 Characterization of ruminal bacteria in grazing Nellore steers Caracterización de bacterias ruminales en novillos Nelore en pastoreo Caraterização bacteriana ruminal em novilhos Nelore em pastejo Raphael B de Jesus1 ; Yury T Granja-Salcedo1* ; Juliana D Messana1 ; Luciano T Kishi2 ; Eliana G M Lemos3 ; Jackson Antonio M de Souza3 ; Telma T Berchielli1. 1Departamento de Zootecnia, Faculdade de Ciências Agrarias e Veterinárias (FCAV), Universidade Estadual Paulista (UNESP), Câmpus Jaboticabal, Jaboticabal SP, Brazil . 2Departamento de Tecnologia, Faculdade de Ciências Agrarias e Veterinárias (FCAV), Universidade Estadual Paulista (UNESP), Câmpus Jaboticabal, Jaboticabal, SP, Brazil . 3Departamento de Biologia, Faculdade de Ciências Agrarias e Veterinárias (FCAV), Universidade Estadual Paulista (UNESP), Câmpus Jaboticabal, Jaboticabal, SP, Brazil . Received: November 7, 2017; accepted: March 5, 2019 To cite this article: De Jesus RB, Granja-Salcedo YT, Messana JD, Kishi LT, Lemos EGM, De Souza JAM, Barchielli TT. Characterization of ruminal bacteria in grazing Nellore steers. Rev Colomb Cienc Pecu 2019; 32(4): 248-260. DOI: https://doi.org/10.17533/udea.rccp.v32n4a01 This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. eISSN: 2256-2958 Rev Colomb Cienc Pecu 2019; 32(4):248-260 Ruminal bacteria in grazing steers 249 Abstract Background: Rumen microorganisms have developed a series of complex interactions, representing one of the best examples of symbiosis between microorganisms in nature. Conventional taxonomic methods based on culture techniques are being replaced by molecular techniques that are faster and more accurate. Objective: To characterize rumen bacterial diversity of Nellore steers grazing on tropical pastures by sequencing the 16S rRNA gene using Illumina sequencing. -

Characterization of Cucumber Fermentation Spoilage Bacteria by Enrichment Culture and 16S Rdna Cloning
Characterization of Cucumber Fermentation Spoilage Bacteria by Enrichment Culture and 16S rDNA Cloning Fred Breidt, Eduardo Medina, Doria Wafa, Ilenys P´erez-D´ıaz, Wendy Franco, Hsin-Yu Huang, Suzanne D. Johanningsmeier, and Jae Ho Kim Abstract: Commercial cucumber fermentations are typically carried out in 40000 L fermentation tanks. A secondary fermentation can occur after sugars are consumed that results in the formation of acetic, propionic, and butyric acids, concomitantly with the loss of lactic acid and an increase in pH. Spoilage fermentations can result in significant economic loss for industrial producers. The microbiota that result in spoilage remain incompletely defined. Previous studies have implicated yeasts, lactic acid bacteria, enterobacteriaceae, and Clostridia as having a role in spoilage fermentations. We report that Propionibacterium and Pectinatus isolates from cucumber fermentation spoilage converted lactic acid to propionic acid, increasing pH. The analysis of 16S rDNA cloning libraries confirmed and expanded the knowledge gained from previous studies using classical microbiological methods. Our data show that Gram-negative anaerobic bacteria supersede Gram-positive Fermincutes species after the pH rises from around 3.2 to pH 5, and propionic and butyric acids are produced. Characterization of the spoilage microbiota is an important first step in efforts to prevent cucumber fermentation spoilage. Keywords: pickled vegetables, Pectinatus, Propionibacteria, secondary cucumber fermentation, spoilage M: Food Microbiology Practical Application: An understanding of the microorganisms that cause commercial cucumber fermentation spoilage & Safety may aid in developing methods to prevent the spoilage from occurring. Introduction cucumbers fermented at 2.3% NaCl (Fleming and others 1989). Commercial cucumber fermentations are typically carried out In this fermentation tank, the initial lactic acid fermentation was in large 40000 L outdoor tanks (reviewed by Breidt and others completed within 2 wk, with 1.2% lactic acid formed (pH 3.6) 2007). -

ROLE of NOVEL QUORUM SENSING MOLECULES (DKPS-DIKETOPIPERAZINES) AS ACTIVATORS of BACTERIAL VIRULENCE and HOST RESPONSE Alex Gill
ROLE OF NOVEL QUORUM SENSING MOLECULES (DKPS-DIKETOPIPERAZINES) AS ACTIVATORS OF BACTERIAL VIRULENCE AND HOST RESPONSE Alex Gillone A thesis submitted to the faculty of the University of North Carolina at Chapel Hill in partial fulfillment of the requirements for the degree of Master of Science in the Department of Periodontology in the School of Dentistry. Chapel Hill 2016 Approved by: Steven Offenbacher Silvana P. Barros Roland Arnold © 2016 Alex Gillone ALL RIGHTS RESERVED ii ABSTRACT Alex Gillone: Role of Novel Quorum sensing molecules (DKPs-Diketopiperazines) as activators of bacterial virulence and host response. (Under the direction of Steven Offenbacher) Objectives: The aim of this project was to establish the functional role of novel quorum sensing molecules (Diketopiperazines – DKPs) on activation of bacterial virulence properties and the potential effects on host cells as activators of the innate immune response. Methods: The effect of DKPs on the growth and virulence properties of the periodontal pathogen, Porphyromonas gingivalis (P.g.) A7436 strain was examined. Secondarily, the effect of DKPs on human monocyte (THP-1) viability, growth and cytokine production upon lipopolysaccharide (LPS) stimulation was determined. THP-1 cells were collected, counted and the cell lysate was evaluated for Interleukin 1β (IL-1β) mRNA expression. Results: Our results demonstrate that DKPs minimally affect the growth of P. g. DKP alone did not significantly alter THP-1 viability (p=0.20), indicating it was not toxic to the cells. However, analysis of the IL-1β mRNA expression indicates that DKP inhibited the inflammatory response of LPS-stimulated THP-1 cells. Conclusions: We have confirmed that DKPs minimally affect the growth of P. -

Outline Release 7 7C
Garrity, et. al., March 6, 2007 Taxonomic Outline of the Bacteria and Archaea, Release 7.7 March 6, 2007. Part 7 – The Bacteria: Phylum “Firmicutes”: Class “Clostridia” George M. Garrity, Timothy G. Lilburn, James R. Cole, Scott H. Harrison, Jean Euzéby, and Brian J. Tindall F Phylum Firmicutes AL N4Lid DOI: 10.1601/nm.3874 Class "Clostridia" N4Lid DOI: 10.1601/nm.3875 71 Order Clostridiales AL Prévot 1953. N4Lid DOI: 10.1601/nm.3876 Family Clostridiaceae AL Pribram 1933. N4Lid DOI: 10.1601/nm.3877 Genus Clostridium AL Prazmowski 1880. GOLD ID: Gi00163. GCAT ID: 000971_GCAT. Entrez genome id: 80. Sequenced strain: BC1 is from a non-type strain. Genome sequencing is incomplete. Number of genomes of this species sequenced 6 (GOLD) 6 (NCBI). N4Lid DOI: 10.1601/nm.3878 Clostridium butyricum AL Prazmowski 1880. Source of type material recommended for DOE sponsored genome sequencing by the JGI: ATCC 19398. High-quality 16S rRNA sequence S000436450 (RDP), M59085 (Genbank). N4Lid DOI: 10.1601/nm.3879 Clostridium aceticum VP (ex Wieringa 1940) Gottschalk and Braun 1981. Source of type material recommended for DOE sponsored genome sequencing by the JGI: ATCC 35044. High-quality 16S rRNA sequence S000016027 (RDP), Y18183 (Genbank). N4Lid DOI: 10.1601/nm.3881 Clostridium acetireducens VP Örlygsson et al. 1996. Source of type material recommended for DOE sponsored genome sequencing by the JGI: DSM 10703. High-quality 16S rRNA sequence S000004716 (RDP), X79862 (Genbank). N4Lid DOI: 10.1601/nm.3882 Clostridium acetobutylicum AL McCoy et al. 1926. Source of type material recommended for DOE sponsored genome sequencing by the JGI: ATCC 824.