Short-Term Intraocular Pressure Changes After Intravitreal Injection
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Acute Keratoconus-Like Corneal Hydrops Secondary to Ocular
perim Ex en l & ta a l ic O p in l h Journal of Clinical & Experimental t C h f a o l m l a o Ke et al., J Clin Exp Ophthalmol 2017, 8:6 n l o r g u y o Ophthalmology J DOI: 10.4172/2155-9570.1000694 ISSN: 2155-9570 Case Report Open Access Acute Keratoconus-Like Corneal Hydrops Secondary to Ocular Massage Following Trabeculectomy Hongmin Ke, Chengguo Zuo and Mingkai Lin* State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, Sun Yat-sen University, 54 Xianlie Nan Road, Guangzhou, China *Corresponding author: Mingkai Lin, State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, 54 Xianlie Nan Road, Guangzhou, China, 510060, E- mail: [email protected] Received date: November 13, 2017; Accepted date: November 21, 2017; Published date: November 23, 2017 Copyright: © 2017 Ke H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract Purpose: To report a case of acute keratoconus-like corneal hydrops in a patient with long-term ocular massage following trabeculectomy. Methods: Case report and review of medical literature. Results: A rare complication of acute keratoconus-like corneal hydrops occurred in a patient following the use of ocular massage to maintain satisfactory aqueous humor filtration after trabeculectomy. The patient had a history of high myopia but denied previous ocular trauma, allergic disease and a family history of keratoconus. Slit-lamp examination demonstrated keratoconus-like corneal hydrops with formation of epithelial microcystic, and intrastromal cleft. -

Endogenous Endophthalmitis: Diagnosis and Treatment
UVEITIS OPHTHALMIC PEARLS Endogenous Endophthalmitis: Diagnosis and Treatment ndogenous endophthalmitis Pathogenesis 1 (EE) is an uncommon intraoc- The infectious agent travels via the Eular infection with potentially bloodstream and multiplies in the cho- devastating visual consequences. An roid, eventually infiltrating the retina endogenous source is responsible for and spreading into the vitreous.4 roughly 2% to 8% of all endophthal- A diagnosis of EE merits a systemic mitis.1 Prompt diagnosis and treatment workup for the source of infection, are essential to obtain the best visual although in 44% of cases no source is outcomes. The underlying infection found.2 EE has been most commonly should also be investigated and man- associated with liver abscesses, sinus aged, although it remains unidentified infections, endocarditis, meningitis, or in many cases. presence of indwelling catheters. Etiology Diagnosis About half of reported EE cases are Presentation. Patient presentation YEAST INFECTION. Fundus photo of caused by bacteria and half by fungi.2 ranges from asymptomatic to symp- a white, fluffy chorioretinal infiltrate In North America and Europe, the toms typical of severe uveitis, including erupting into the vitreous. Vitreous fluid most frequently identified causative a red, painful eye with photophobia, grew Candida albicans. bacteria are Staphylococcus aureus and floaters, or reduced vision. Although EE Streptococcus pneumoniae, while in East is most often unilateral, up to a third of conditions, infectious and noninfec- Asia, Klebsiella pneumoniae is chiefly cases have bilateral involvement.5 tious, should be considered in formu- responsible.1 Among fungal etiologies, Ocular examination. Symptomat- lating the diagnosis. See “Differential Candida albicans (Fig. 1) is the most ic patients may have reduced visual Diagnosis” on the next page. -

Shift Work: a Risk Factor for Central Serous Chorioretinopathy
Shift Work: A Risk Factor for Central Serous Chorioretinopathy ELODIE BOUSQUET, MYRIAM DHUNDASS, MATHIEU LEHMANN, PIERRE-RAPHAE¨L ROTHSCHILD, VIRGINIE BAYON, DAMIEN LEGER, CIARA BERGIN, ALI DIRANI, TALAL BEYDOUN, AND FRANCINE BEHAR-COHEN PURPOSE: To investigate if shift work or sleep distur- and focal serous detachments of the neurosensory retina bances are risk factors for central serous chorioretinop- and retinal pigment epithelium alterations. The role of athy (CSCR). choroid hyperpermeability in the pathogenesis of CSCR DESIGN: Prospective case-control study. has been well documented recently with multimodal imag- 2 METHODS: Forty patients with active CSCR and 40 ing modalities. In CSCR patients, a thick choroid has controls (age- and sex-matched) were prospectively been reported not only in the affected eye but also in the recruited from the Ophthalmology Department of Hoˆtel fellow eye, which is consistent with bilateral choroidal Dieu Hospital, Paris, between November 2013 and hyperpermeability.2,3 December 2014. All patients were asked to complete a To date, several risk factors for CSCR have been questionnaire addressing previously described risk factors identified,3 and the most consistent is corticosteroid and working hours, as well as the Insomnia Severity exposure from therapeutic administration or from endog- Index (ISI), a validated instrument for assessing sleep enous overproduction, as in Cushing syndrome.4–6 disturbances. Corticosteroids were recently shown to induce RESULTS: The mean age of the CSCR group was 44 -

The Efficacy of Intravitreal Conbercept for Chronic Central Serous Chorioretinopathy
Hindawi Journal of Ophthalmology Volume 2019, Article ID 7409426, 5 pages https://doi.org/10.1155/2019/7409426 Research Article The Efficacy of Intravitreal Conbercept for Chronic Central Serous Chorioretinopathy Jianbo Mao,1 Caiyun Zhang,1 Chenyi Liu,2 Lijun Shen ,1 Jimeng Lao,1 Yirun Shao,1 Yiqi Chen,1 and Jiwei Tao1 1Eye Hospital of Wenzhou Medical University, Wenzhou, Zhejiang, China 2Chicago College of Optometry, Midwestern University, Downers Grove, IL, USA Correspondence should be addressed to Lijun Shen; [email protected] Received 11 February 2019; Revised 10 April 2019; Accepted 25 April 2019; Published 7 May 2019 Academic Editor: Elad Moisseiev Copyright © 2019 Jianbo Mao et al. (is is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Purpose. To evaluate the efficacy and safety of conbercept for patients with chronic central serous chorioretinopathy (CSC). Methods. A retrospective clinical study. (irty-one patients (35 eyes) with chronic CSC were given intravitreal injections of conbercept and followed up for at least 6 months. Observed indicators included best-corrected visual acuity (BCVA), central macular thickness (CMT), and resolution of subretinal fluid (SRF). Serial changes in BCVA and CMT were analyzed by using repeated measures analysis of variance. Results. During the 6-month follow-up, the mean number of injections required and performed was 1.77 ± 0.60. (e logMAR BCVA was 0.48 ± 0.26 at the baseline, 0.34 ± 0.26, 0.30 ± 0.26, 0.27 ± 0.26, 0.24 ± 0.26, and 0.23 ± 0.26 at 2-week and 1-, 2-, 3-, and 6-month follow-ups, respectively (F � 27.173, P < 0:05). -

Endophthalmitis
RETINA HEALTH SERIES | Facts from the ASRS The Foundation American Society of Retina Specialists Committed to improving the quality of life of all people with retinal disease. Endophthalmitis is an infection inside the eye SIGNS AND SYMPTOMS that can either be acute or chronic, meaning that it can develop very rapidly which is most common, or develop Endophthalmitis causes the white slowly and persist for long periods of time of the eye to be inflamed. There may be a white or yellow discharge on or inside the eyelid, and the cornea may show a white cloud- iness. There may also be a layer of white cells (hypopyon) present within the anterior chamber of the eye between the iris and the cornea. (Figure 1) Endophthalmitis is usually a very serious problem and prompt examination by an ophthalmologist is essential to make an appropriate diagnosis and initiate treatment. Other symptoms include: • Eye pain and redness • Decreased vision • Trouble looking at bright lights (photophobia), usually sudden onset Figure 1 Hypopyon is an accumulation of white blood cells in the anterior chamber of the eye and corneal WHAT IS THE RETINA? infiltrate associated with infectious endophthalmitis. Image courtesy of ©Retina Image Bank, contributed by Aleksandra V. Rachitskay, MD, Cole Eye Institute, Cleveland Clinic. 2014. Image 16250. Causes: Acute cases of endophthalmitis are caused by gram-positive (or less frequently gram-negative) bacteria and are most often seen within 6 weeks after surgery or trauma to the eye. Chronic cases that occur outside of the 6-week window are often related to a previous surgery and are commonly caused by slowly progressive infections such as Propionibacterium acnes or fungus. -

Treatment of Postkeratitis Fusarium Endophthalmitis with Amphotericin B Lipid Complex
Cornea 19(6): 853–856, 2000. © 2000 Lippincott Williams & Wilkins, Inc., Philadelphia Treatment of Postkeratitis Fusarium Endophthalmitis with Amphotericin B Lipid Complex David Goldblum, M.D., Beatrice E. Frueh, M.D., Stefan Zimmerli, M.D., and Matthias Bo¨hnke, M.D. Purpose. The authors report the first case of Fusarium solani tients treated with amphotericin B develop some degree of renal keratitis that progressed to fungal endophthalmitis and was suc- impairment. Patients who receive a cumulated dose of more than cessfully treated with amphotericin B lipid complex (ABLC). 4–5 g of the drug are particularly at risk.5 Method. The case of a 34-year-old immunocompetent woman Three new lipid formulations of amphotericin B with reduced who developed a contact lens-related F. solani keratitis requiring nephrotoxicity are now available for clinical use. Amphotericin B emergency penetrating keratoplasty (PKP) was analyzed. The im- lipid complex (ABLC) (Abelcet®; The Liposome Company, munocompetent patient developed fungal endophthalmitis (ante- rior chamber tap positive for F. solani three months after PKP) and Princeton, NJ, U.S.A.) is a concentration of ribbon-like structures was eventually treated with ABLC. Results. Systemic amphoteri- of a bilayered membrane formed by combining a 7:3 ratio of cin B (total, 0.42 g) and ketoconazole in addition to topical nata- dimyristoyl phosphatidylcholine and dimyristoyl phosphatidyl- mycin and amphotericin did not prove to be effective in eradicat- glycerol with amphotericin B. Amphotericin B colloidal dispersion ing the mycosis in the anterior chamber. Under ABLC treatment (Amphocil®; Sequus Pharmaceuticals, Menlo Park, CA, U.S.A.) (total, 8.79 g), the anterior chamber inflammation resolved com- is composed of disc-like structures of cholesteryl sulfate com- pletely. -

Incidence of Post-Cataract Endophthalmitis at Aravind Eye
ARTICLE Incidence of post-cataract endophthalmitis at Aravind Eye Hospital Outcomes of more than 42000 consecutive cases using standardized sterilization and prophylaxis protocols Ravilla D. Ravindran, MS, DO, Rengaraj Venkatesh, DO, DNB, David F. Chang, MD, Sabyasachi Sengupta, DO, Jamyang Gyatsho, MS, Badrinath Talwar, MS, DNB PURPOSE: To report the incidence of postoperative endophthalmitis at a high-volume eye hospital in southern India using a modified cost-effective sterilization protocol. SETTING: Aravind Eye Hospital and Post Graduate Institute of Ophthalmology, Pondicherry, India. METHODS: In this retrospective observational series at a single eye hospital, records of patients who had cataract surgery using a modified sterilization protocol from January 2007 through August 2008 and developed postoperative endophthalmitis within the first 3 postoperative months were drawn from a computerized database. The patient’s socioeconomic status, the surgeon’s experi- ence, and the type of cataract procedure performed were analyzed as possible risk factors using the chi-square test/Fischer exact test. RESULTS: During the study period, 42426 cataract surgeries were performed. From these, 38 cases of presumed postoperative endophthalmitis were identified (incidence 0.09%). Thirty-five of the 38 cases were in the manual large- and small-incision extracapsular cataract extraction (ECCE) group, which had a statistically higher rate than the phacoemulsification group (P Z .016). There was no sta- tistical difference in the endophthalmitis rates between private patients and charity patients for either surgical method (manual ECCE or phacoemulsification). CONCLUSIONS: The modified sterilization and asepsis protocol adopted to facilitate high-volume cataract surgery in a clinical setting appeared to be safe and effective in preventing postsurgical endophthalmitis. -

A Spectral Domain Optical Coherence Tomography Study
Eye (2017) 31, 1488–1495 © 2017 Macmillan Publishers Limited, part of Springer Nature. All rights reserved 0950-222X/17 www.nature.com/eye 1 1 1 1 1 CLINICAL STUDY Atrophy of retinal XLu, W Chen ,HXia, K Zheng , C Jin , DSC Ng2 and H Chen1 inner layers is associated with poor vision after endophthalmitis: a spectral domain optical coherence tomography study Abstract Purpose To investigate the retinal structural can be open globe trauma, intraocular surgery, changes in endophthalmitis and their keratitis, or endogenous.1 The reported association with visual outcome. incidence of endophthalmitis is 2.1% after open Patients and methods Forty-five eyes of globe injury,2 0.06 ~ 0.2% after cataract surgery,3 45 patients diagnosed with endophthalmitis 0.039% after vitrectomy,4 and 0.049% after were included. Spectral domain optical intravitreal injection.5 Although endophthalmitis coherence tomography (SD-OCT) was can be adequately managed with intravitreal fl performed after in ammation was controlled. injection of antibiotics and/or pars plana The relationship between SD-OCT features vitrectomy, impairment of vision has been and best-corrected visual acuity (BCVA) at the observed in some patients.6 This could be due to last follow-up was analyzed. damage of the retinal structure. Results The structural changes included Spectral domain optical coherence 1Joint Shantou inner segment ellipsoid (ISe) disruption tomography (SD-OCT) allows visualization of International Eye Center, (49%), atrophy of retinal inner layers (24%), the cross-sectional structure of retina in vivo.7 Shantou University, epimacular membrane (24%), and macular It is now widely being used to investigate the Chinese University of Hong edema (24%). -

Dry Eye Disease Management for IMPROVING PATIENT OUTCOMES
CME MONOGRAPH Visit https://tinyurl.com/DEDOutcomesCME for online testing and instant CME certificate. CURRENT PERSPECTIVES Dry Eye Disease Management FOR IMPROVING PATIENT OUTCOMES FACULTY TERRY KIM, MD (CHAIR) • ROSA BRAGA-MELE, MD, MEd, FRCSC JESSICA CIRALSKY, MD • CHRISTOPHER J. RAPUANO, MD Original Release: May 1, 2019 • Expiration: May 31, 2020 This continuing medical education activity is jointly provided by New York Eye and Ear Infirmary of Mount Sinai and MedEdicus LLC. This continuing medical education activity is supported through an unrestricted educational grant from Shire. Distributed with LEARNING METHOD AND MEDIUM Jessica Ciralsky, MD, had a financial agreement or affiliation during the This educational activity consists of a supplement and ten (10) study past year with the following commercial interests in the form of Consultant/ questions. The participant should, in order, read the learning objectives Advisory Board: Allergan; and Shire. contained at the beginning of this supplement, read the supplement, Terry Kim, MD, had a financial agreement or affiliation during the past answer all questions in the post test, and complete the Activity year with the following commercial interests in the form of Consultant/ Evaluation/Credit Request form. To receive credit for this activity, Advisory Board: Actavis; Aerie Pharmaceuticals, Inc; Alcon; Allergan; please follow the instructions provided on the post test and Activity Avedro, Inc; Avellino Labs; Bausch & Lomb Incorporated; BlephEx; Evaluation/Credit Request form. This educational -

CORNEAL ULCERS Diagnosis and Management
CORNEAL ULCERS Diagnosis and Management System requirement: • Windows XP or above • Power DVD player (Software) • Windows Media Player 10.0 version or above • Quick time player version 6.5 or above Accompanying DVD ROM is playable only in Computer and not in DVD player. Kindly wait for few seconds for DVD to autorun. If it does not autorun then please do the following: • Click on my computer • Click the drive labelled JAYPEE and after opening the drive, kindly double click the file Jaypee CORNEAL ULCERS Diagnosis and Management Namrata Sharma MD DNB MNAMS Associate Professor of Ophthalmology Cornea, Cataract and Refractive Surgery Services Dr. Rajendra Prasad Centre for Ophthalmic Sciences All India Institute of Medical Sciences, New Delhi India Rasik B Vajpayee MS FRCSEd FRANZCO Head, Corneal and Cataract Surgery Centre for Eye Research Australia Royal Victorian Eye and Ear Hospital University of Melbourne Australia Forewords Hugh R Taylor Peter R Laibson ® JAYPEE BROTHERS MEDICAL PUBLISHERS (P) LTD New Delhi • Ahmedabad • Bengaluru • Chennai • Hyderabad • Kochi • Kolkata • Lucknow • Mumbai • Nagpur Published by Jitendar P Vij Jaypee Brothers Medical Publishers (P) Ltd B-3 EMCA House, 23/23B Ansari Road, Daryaganj New Delhi 110 002, India Phones: +91-11-23272143, +91-11-23272703, +91-11-23282021, +91-11-23245672 Rel: +91-11-32558559, Fax: +91-11-23276490, +91-11-23245683 e-mail: [email protected] Visit our website: www.jaypeebrothers.com Branches • 2/B, Akruti Society, Jodhpur Gam Road Satellite Ahmedabad 380 015, Phones: +91-79-26926233, -
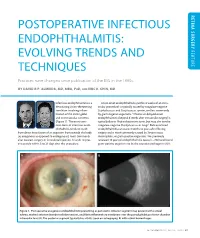
Postoperative Infectious Endophthalmitis: Evolving Trends and Feature Techniques
RETINA SURGERY RETINA POSTOPERATIVE INFECTIOUS ENDOPHTHALMITIS: EVOLVING TRENDS AND FEATURE TECHNIQUES Practices have changed since publication of the EVS in the 1990s. BY DAVID R.P. ALMEIDA, MD, MBA, PHD, AND ERIC K. CHIN, MD Infectious endophthalmitis is a Acute-onset endophthalmitis (within 6 weeks of an intra- devastating vision-threatening ocular procedure) is typically caused by coagulase-negative condition involving inflam- Staphylococcus and Streptococcus species, and less commonly mation of the entire globe by gram-negative organisms.1 Chronic or delayed-onset and its intraocular contents endophthalmitis (beyond 6 weeks after intraocular surgery) is (Figure 1). The most com- typically due to Propionibacterium acnes, but may also involve mon form of infectious endo- coagulase-negative Staphylococcus or fungi.2 Bleb-associated phthalmitis tends to result endophthalmitis can occur months to years after filtering from direct inoculation of an organism from outside the body surgery and is most commonly caused by Streptococcus, (ie, exogenous as opposed to endogenous), most commonly Haemophilus, or gram-positive organisms. We previously after cataract surgery or intravitreal injection. It tends to pres- reviewed 10 years of endophthalmitis cases (n = 758) and found ent acutely within 3 to 21 days after the procedure. gram-positive organisms to be the causative pathogen in 80% A B Figure 1. Postoperative exogenous endophthalmitis presenting as panuveitis. Anterior segment may present with corneal edema, marked anterior chamber cellular reaction, and fibrin inflammatory membrane over the pseudophakic posterior chamber intraocular lens (A). The posterior segment typically has vitritis (seen on echography; B) with retinal hemorrhages. SEPTEMBER 2016 | RETINA TODAY 27 FEATURE RETINA SURGERY RETINA Figure 2. -

Complex Endophthalmitis and Phacoallergic Uveitis
Br J Ophthalmol: first published as 10.1136/bjo.62.2.105 on 1 February 1978. Downloaded from British Journal of Ophthalmology, 1978, 62, 105-109 Release of prostaglandins in experimental immune- complex endophthalmitis and phacoallergic uveitis AMJAD RAHI, PRIMAL BHATTACHERJEE, AND ROOPNARAIN MISRA From the Department of Pathology and Experimental Ophthalmology, Institute of Ophthalmology, University of London SUMMARY Prostaglandins-E were demonstrated in the aqueous humour, the anterior uvea, and the choroid of rabbits in which type III allergic reactions were produced by two different methods. In general the levels of prostaglandins were found to be higher in those animals in which the immune complexes were formed from autologous rather than heterologous tissue antigens. Autocoids are endogenous autopharmacological (Bhattacherjee, 1977; Bhattacherjee and Phylactos, agents which are intimately concerned with the 1977). It is also well established that prostaglandins, general inflammatory processes in the eye and else- particularly the E-type, are released in a variety of where in the body. One group of autocoids, the experimental and clinical allergic conditions (Giroud prostaglandins (PGs), belong to a family of 20- and Willoughby, 1970; Brocklehurst, 1975). Since carbon unsaturated fatty acids, and although they at least 4 distinct types of allergic reactions, each were first discovered several decades ago (Goldblatt, with a different pathogenetic mechanism, have been copyright. 1933) it is only recently that prostaglandins have recognised,