Carbon Dioxide 1 Carbon Dioxide
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

On the Formation of Higher Carbon Oxides in Extreme Environments
Chemical Physics Letters 465 (2008) 1–9 Contents lists available at ScienceDirect Chemical Physics Letters journal homepage: www.elsevier.com/locate/cplett FRONTIERS ARTICLE On the formation of higher carbon oxides in extreme environments Ralf I. Kaiser a,*, Alexander M. Mebel b a Department of Chemistry, University of Hawaii at Manoa, 2545 The Mall (Bil 301A), Honolulu, HI 96822, USA b Department of Chemistry and Biochemistry, Florida International University, Miami, FL 33199, USA article info abstract Article history: Due to the importance of higher carbon oxides of the general formula COx (x > 2) in atmospheric chem- Received 15 May 2008 istry, isotopic enrichment processes, low-temperature ices in the interstellar medium and in the outer In final form 22 July 2008 solar system, as well as potential implications to high-energy materials, an overview on higher carbon Available online 29 July 2008 oxides COx (x = 3–6) is presented. This article reviews recent developments on these transient species. Future challenges and directions of this research field are highlighted. Ó 2008 Elsevier B.V. All rights reserved. 1. Introduction tion has no activation energy, proceeds with almost unit efficiency, and most likely involves a reaction intermediate. However, neither In recent years, the interest in carbon oxides of higher complex- reaction products (kinetics studies) nor the nature of the interme- 1 + ityP than carbon monoxide (CO; X R ) and carbon dioxide (CO2; diate (kinetics and dynamicsP studies) were determined. On the 1 þ 1 + 3 À X g ) of the generic formula COx (x > 2) has been fueled by com- other hand, a CO(X R )+O2(X g ) exit channel was found to have plex reaction mechanisms of carbon oxides with atomic oxygen in an activation energy between 15 and 28 kJ molÀ1 in the range of the atmospheres of Mars [1–4] and Venus [5]. -

Briefing on Carbon Dioxide Specifications for Transport 1St Report of the Thematic Working Group On: CO2 Transport, Storage
Briefing on carbon dioxide specifications for transport 1st Report of the Thematic Working Group on: CO2 transport, storage and networks Release Status: FINAL Author: Dr Peter A Brownsort Date: 29th November 2019 Filename and version: Briefing-CO2-Specs-FINAL-v1.docx EU CCUS PROJECTS NETWORK (No ENER/C2/2017-65/SI2.793333) This project is financed by the European Commission under service contract No. ENER/C2/2017-65/SI2.793333 1 About the CCUS Projects Network The CCUS Projects Network comprises and supports major industrial projects underway across Europe in the field of carbon capture and storage (CCS) and carbon capture and utilisation (CCU). Our Network aims to speed up delivery of these technologies, which the European Commission recognises as crucial to achieving 2050 climate targets. By sharing knowledge and learning from each other, our project members will drive forward the delivery and deployment of CCS and CCU, enabling Europe’s member states to reduce emissions from industry, electricity, transport and heat. http://www.ccusnetwork.eu/ © European Union, 2019 No third-party textual or artistic material is included in the publication without the copyright holder’s prior consent to further dissemination by other third parties. Reproduction is authorised provided the source is acknowledged. This project is financed by the European Commission under service contract No. ENER/C2/2017-65/SI2.793333 2 (Intentionally blank) This project is financed by the European Commission under service contract No. ENER/C2/2017-65/SI2.793333 3 Executive summary There are two types of specification generally relevant to carbon dioxide (CO2) transport, the product specification for end use and the requirement specification for transport. -

Carbon Dioxide (Refrigerated Liquid)
Carbon dioxide, refrigerated liquid Safety Data Sheet P-4573 This SDS conforms to U.S. Code of Federal Regulations 29 CFR 1910.1200, Hazard Communication. Date of issue: 01/01/1997 Revision date: 01/30/2021 Supersedes: 09/08/2020 Version: 2.0 SECTION: 1. Product and company identification 1.1. Product identifier Product form : Substance Substance name : Carbon dioxide, refrigerated liquid CAS-No. : 124-38-9 Formula : CO2 Other means of identification : Liquiflow Liquid Carbon Dioxide, Medipure Liquid Carbon Dioxide 1.2. Relevant identified uses of the substance or mixture and uses advised against Use of the substance/mixture : Medical applications. Industrial use Food applications. 1.3. Details of the supplier of the safety data sheet Linde Inc. 10 Riverview Drive Danbury, CT 06810-6268 - USA 1.4. Emergency telephone number Emergency number : Onsite Emergency: 1-800-645-4633 CHEMTREC, 24hr/day 7days/week — Within USA: 1-800-424-9300, Outside USA: 001-703-527-3887 (collect calls accepted, Contract 17729) SECTION 2: Hazard identification 2.1. Classification of the substance or mixture GHS US classification Simple asphyxiant Press. Gas (Ref. Liq.) H281 2.2. Label elements GHS US labeling Hazard pictograms (GHS US) : GHS04 Signal word (GHS US) : Warning Hazard statements (GHS US) : H281 - CONTAINS REFRIGERATED GAS; MAY CAUSE CRYOGENIC BURNS OR INJURY OSHA-H01 - MAY DISPLACE OXYGEN AND CAUSE RAPID SUFFOCATION. CGA-HG03 - MAY INCREASE RESPIRATION AND HEART RATE. Precautionary statements (GHS US) : P202 - Do not handle until all safety precautions have been read and understood. P271+P403 - Use and store only outdoors or in a well-ventilated place. -
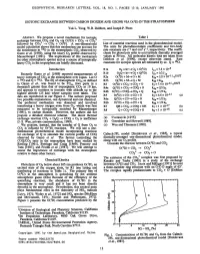
Isotopic Exchange Between Carbon Dioxide and Ozone Via O1D in The
GEOPHYSICAL RESEARCH LETTERS, VOL. 18, NO. 1, PAGES 13-16, JANUARY 1991 ISOTOPICEXCHANGE BETWEEN CARBONDIOXIDE AND OZONEVIA O(!D) IN THE STRATOSPHERE Yuk L. Yung, W.B. DoMore,and Joseph P. Pinto Abstract. We proposea novelmechanism for isotopic Table 1 exchangebetween CO 2 and0 3 vi•aO(ID) + CO2 -->CO_•* followedby CO3' --> CO2 + O(aP). A one-•ensior•al List of essentialreactions used in the photochemicalmodel. model calculation shows that this mechanism can account for The units for photodissociationcoefficients and two-body theenrichment in 180 in thestratospheric CO2 observed by rateconstants are s -1 andcm 3 s-l, respectively.The coeffi- Gatnoeta!. [1989],using the heavy 0 3 profile-observed by cientsfor photolysisrefer to nfid-latitudedimally averaged Mauersberger[1981]. The implicationsof this mechanism values at 30 kan. All molecular kinetic data are taken frmn for otherstratospheric species and as a sourceof isotopically DoMore etal. [1990], except otherwise stated. Rate heavyCO 2 in thetroposphere are briefly discussed. constantsforisotopic species are estimated byus. Q = 180. h•troduction Rla O3+ bY-->O2 + O(1D) Jla=7.4x10 -5 RecentlyGamo et at. [1989] reportedmeasurements of Rib O2Q+ hv-->O2 + Q(!D) Jlb=1/2 J la heavyisotopes of CO2 in the stratosphereover Japan. Let O R2a O(ID)+ M--->O+ M k2a= 2.0x 10-11 e ll0/T = 160 andQ = 180.The fiQ of stratosphericCO2, as defined R2b Q(1D) + M --->Q + M k2b= k2a in Gatno et at., was found to be 2O/oo(two parts in a R3 O(ID)+ CO2---->CO2 + O k3 = 7.4x 10-11 e 120/T thousand)greater than that of troposphericCO 2 at 19 km, R4a Q(1D)+ CO2-->CO Q + O k4a= 2/3k 3 and appearsto continueto increasewith altitudeup to the R4b O(ID)+ COQ-->CO2 + Q k4b= 1/3k 3 highestaltitude (25 km) where sampleswere taken. -

Liquid Carbon Dioxide (CO2) SDS (Safety Data Sheet) P-4573-D
Product: Carbon Dioxide, Refrigerated Liquid P-4573-D Date: December 2009 Praxair Material Safety Data Sheet 1. Chemical Product and Company Identification Product Name: Carbon dioxide, refrigerated liquid Trade Names: Liquiflow™ Liquid Carbon (MSDS No. P-4573-D) Dioxide, Medipure® Liquid Carbon Dioxide Chemical Name: Carbon dioxide Synonyms: Carbon dioxide (cryogenic liquid), LCO2, liquefied CO2 Chemical Family: Acid anhydride Product Grades: Industrial, USP Telephone: Emergencies: 1-800-645-4633* Company Name: Praxair, Inc. CHEMTREC: 1-800-424-9300* 39 Old Ridgebury Road Routine: 1-800-PRAXAIR Danbury, CT 06810-5113 * Call emergency numbers 24 hours a day only for spills, leaks, fire, exposure, or accidents involving this product. For routine information, contact your supplier, Praxair sales representative, or call 1-800-PRAXAIR (1-800-772-9247). 2. Hazards Identification EMERGENCY OVERVIEW WARNING! Cold liquid and gas under pressure. Can cause rapid suffocation. Can increase respiration and heart rate. May cause nervous system damage. May cause frostbite. May cause dizziness and drowsiness. Self-contained breathing apparatus and protective clothing may be required by rescue workers. This product is a colorless, odorless liquid that transforms to white crystalline particles when discharged from its container. The gas is slightly acidic and may be felt to have a slight, pungent odor and biting taste. OSHA REGULATORY STATUS: This material is considered hazardous by the OSHA Hazard Communications Standard (29 CFR 1910.1200). POTENTIAL HEALTH EFFECTS: Effects of a Single (Acute) Overexposure Inhalation. Carbon dioxide gas is an asphyxiant with effects due to lack of oxygen. It is also physiologically active, affecting circulation and breathing. -

Landolt-Börnstein Indexes of Organic Compounds Subvolumes A-I by V
Landolt-Börnstein Indexes of Organic Compounds Subvolumes A-I By V. Vill, C. Bauhofer, G. Peters, H. Sajus, P. Weigner, LCI-Publisher and Chemistry Department of the University of Hamburg All printed index material has been used to build up the comprehensive Scidex database index developed by LCI Publisher GmbH, Hamburg For further information please visit www.lci-publisher.com From this database a CD-ROM and two online versions were derived. The first is attached to each of the printed subvolumes and the latter are offered for free use at the following addresses: Scidex Database online with graphical structure search on http://lb.chemie.uni-hamburg.de/ Or the easy to use html version on http://lb.chemie.uni-hamburg.de/static/ Landolt-Börnstein Numerical Data and Functional Relationships in Science and Technology New Series / Editor in Chief: W. Martienssen Index of Organic Compounds Subvolume A Compounds with 1 to 7 Carbon Atoms Editor: V. Vill Authors: V. Vill, G. Peters, H. Sajus 1 3 ISBN 3-540-66203-0 Springer-Verlag Berlin Heidelberg New York Library of Congress Cataloging in Publication Data Zahlenwerte und Funktionen aus Naturwissenschaften und Technik, Neue Serie Editor in Chief: W. Martienssen Index of Organic Compounds A: Editor: V. Vill At head of title: Landolt-Börnstein. Added t.p.: Numerical data and functional relationships in science and technology. Tables chiefly in English. Intended to supersede the Physikalisch-chemische Tabellen by H. Landolt and R. Börnstein of which the 6th ed. began publication in 1950 under title: Zahlenwerte und Funktionen aus Physik, Chemie, Astronomie, Geophysik und Technik. -

The Structure and Antioxidant Properties
materials Review Recent Developments in Effective Antioxidants: The Structure and Antioxidant Properties Monika Parcheta 1 , Renata Swisłocka´ 1,* , Sylwia Orzechowska 2,3 , Monika Akimowicz 4 , Renata Choi ´nska 4 and Włodzimierz Lewandowski 1 1 Department of Chemistry, Biology and Biotechnology, Bialystok University of Technology, Wiejska 45E, 15-351 Bialystok, Poland; [email protected] (M.P.); [email protected] (W.L.) 2 Solaris National Synchrotron Radiation Centre, Jagiellonian University, Czerwone Maki 98, 30-392 Krakow, Poland; [email protected] 3 M. Smoluchowski Institute of Physics, Jagiellonian University, Łojasiewicza 11, 30-348 Kraków, Poland 4 Prof. Waclaw Dabrowski Institute of Agriculture and Food Biotechnology–State Research Institute, Rakowiecka 36, 02-532 Warsaw, Poland; [email protected] (M.A.); [email protected] (R.C.) * Correspondence: [email protected] Abstract: Since the last few years, the growing interest in the use of natural and synthetic antioxidants as functional food ingredients and dietary supplements, is observed. The imbalance between the number of antioxidants and free radicals is the cause of oxidative damages of proteins, lipids, and DNA. The aim of the study was the review of recent developments in antioxidants. One of the crucial issues in food technology, medicine, and biotechnology is the excess free radicals reduction to obtain healthy food. The major problem is receiving more effective antioxidants. The study aimed to analyze the properties of efficient antioxidants and a better understanding of the molecular ´ Citation: Parcheta, M.; Swisłocka, R.; mechanism of antioxidant processes. Our researches and sparing literature data prove that the Orzechowska, S.; Akimowicz, M.; ligand antioxidant properties complexed by selected metals may significantly affect the free radical Choi´nska,R.; Lewandowski, W. -

~ Coal Mining in Canada: a Historical and Comparative Overview
~ Coal Mining in Canada: A Historical and Comparative Overview Delphin A. Muise Robert G. McIntosh Transformation Series Collection Transformation "Transformation," an occasional paper series pub- La collection Transformation, publication en st~~rie du lished by the Collection and Research Branch of the Musee national des sciences et de la technologic parais- National Museum of Science and Technology, is intended sant irregulierement, a pour but de faire connaitre, le to make current research available as quickly and inex- plus vite possible et au moindre cout, les recherches en pensively as possible. The series presents original cours dans certains secteurs. Elle prend la forme de research on science and technology history and issues monographies ou de recueils de courtes etudes accep- in Canada through refereed monographs or collections tes par un comite d'experts et s'alignant sur le thenne cen- of shorter studies, consistent with the Corporate frame- tral de la Societe, v La transformation du CanadaLo . Elle work, "The Transformation of Canada," and curatorial presente les travaux de recherche originaux en histoire subject priorities in agricultural and forestry, communi- des sciences et de la technologic au Canada et, ques- cations and space, transportation, industry, physical tions connexes realises en fonction des priorites de la sciences and energy. Division de la conservation, dans les secteurs de: l'agri- The Transformation series provides access to research culture et des forets, des communications et de 1'cspace, undertaken by staff curators and researchers for develop- des transports, de 1'industrie, des sciences physiques ment of collections, exhibits and programs. Submissions et de 1'energie . -

Gas-Phase Boudouard Disproportionation Reaction Between Highly Vibrationally Excited CO Molecules
Chemical Physics 330 (2006) 506–514 www.elsevier.com/locate/chemphys Gas-phase Boudouard disproportionation reaction between highly vibrationally excited CO molecules Katherine A. Essenhigh, Yurii G. Utkin, Chad Bernard, Igor V. Adamovich *, J. William Rich Nonequilibrium Thermodynamics Laboratories, Department of Mechanical Engineering, The Ohio State University, Columbus, OH 43202, USA Received 3 September 2006; accepted 21 September 2006 Available online 30 September 2006 Abstract The gas-phase Boudouard disproportionation reaction between two highly vibrationally excited CO molecules in the ground elec- tronic state has been studied in optically pumped CO. The gas temperature and the CO vibrational level populations in the reaction region, as well as the CO2 concentration in the reaction products have been measured using FTIR emission and absorption spectroscopy. The results demonstrate that CO2 formation in the optically pumped reactor is controlled by the high CO vibrational level populations, rather than by CO partial pressure or by flow temperature. The disproportionation reaction rate constant has been determined from the measured CO2 and CO concentrations using the perfectly stirred reactor (PSR) approximation. The reaction activation energy, 11.6 ± 0.3 eV (close to the CO dissociation energy of 11.09 eV), was evaluated using the statistical transition state theory, by comparing the dependence of the measured CO2 concentration and of the calculated reaction rate constant on helium partial pressure. The dispro- À18 3 portionation reaction rate constant measured at the present conditions is kf =(9±4)· 10 cm /s. The reaction rate constants obtained from the experimental measurements and from the transition state theory are in good agreement. -

A Two-Step Graph Convolutional Decoder for Molecule Generation
A Two-Step Graph Convolutional Decoder for Molecule Generation Xavier Bresson Thomas Laurent School of Computer Science and Engineering Department of Mathematics NTU, Singapore Loyola Marymount University [email protected] [email protected] Abstract We propose a simple auto-encoder framework for molecule generation. The molec- ular graph is first encoded into a continuous latent representation z, which is then decoded back to a molecule. The encoding process is easy, but the decoding pro- cess remains challenging. In this work, we introduce a simple two-step decoding process. In a first step, a fully connected neural network uses the latent vector z to produce a molecular formula, for example CO2 (one carbon and two oxygen atoms). In a second step, a graph convolutional neural network uses the same latent vector z to place bonds between the atoms that were produced in the first step (for example a double bond will be placed between the carbon and each of the oxygens). This two-step process, in which a bag of atoms is first generated, and then assembled, provides a simple framework that allows us to develop an efficient molecule auto-encoder. Numerical experiments on basic tasks such as novelty, uniqueness, validity and optimized chemical property for the 250k ZINC molecules demonstrate the performances of the proposed system. Particularly, we achieve the highest reconstruction rate of 90.5%, improving the previous rate of 76.7%. We also report the best property improvement results when optimization is constrained by the molecular distance between the original and generated molecules. 1 Introduction A fundamental problem in drug discovery and material science is to design molecules with arbitrary optimized chemical properties. -
![Alder Reactions of [60]Fullerene with 1,2,4,5-Tetrazines and Additions to [60]Fullerene-Tetrazine Monoadducts](https://docslib.b-cdn.net/cover/7595/alder-reactions-of-60-fullerene-with-1-2-4-5-tetrazines-and-additions-to-60-fullerene-tetrazine-monoadducts-557595.webp)
Alder Reactions of [60]Fullerene with 1,2,4,5-Tetrazines and Additions to [60]Fullerene-Tetrazine Monoadducts
University of New Hampshire University of New Hampshire Scholars' Repository Doctoral Dissertations Student Scholarship Spring 2002 Diels -Alder reactions of [60]fullerene with 1,2,4,5-tetrazines and additions to [60]fullerene-tetrazine monoadducts Mark Christopher Tetreau University of New Hampshire, Durham Follow this and additional works at: https://scholars.unh.edu/dissertation Recommended Citation Tetreau, Mark Christopher, "Diels -Alder reactions of [60]fullerene with 1,2,4,5-tetrazines and additions to [60]fullerene-tetrazine monoadducts" (2002). Doctoral Dissertations. 81. https://scholars.unh.edu/dissertation/81 This Dissertation is brought to you for free and open access by the Student Scholarship at University of New Hampshire Scholars' Repository. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of University of New Hampshire Scholars' Repository. For more information, please contact [email protected]. INFORMATION TO USERS This manuscript has been reproduced from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are reproduced by sectioning the original, beginning at the upper left-hand comer and continuing from left to right in equal sections with small overlaps. -

Mine Rescue Team Training: Metal and Nonmetal Mines (MSHA 3027, Formerly IG 6)
Mine Rescue Team Training Metal and Nonmetal Mines U.S. Department of Labor Mine Safety and Health Administration National Mine Health and Safety Academy MSHA 3027 (Formerly IG 6) Revised 2008 Visit the Mine Safety and Health Administration website at www.msha.gov CONTENTS Introduction Your Role as an Instructor Overview Module 1 – Surface Organization Module 2 – Mine Gases Module 3 – Mine Ventilation Module 4 – Exploration Module 5 – Fires, Firefighting, and Explosions Module 6 – Rescue of Survivors and Recovery of Bodies Module 7 – Mine Recovery Module 8 – Mine Rescue Training Activities Introduction Throughout history, miners have traveled underground secure in the knowledge that if disaster strikes and they become trapped in the mine, other miners will make every possible attempt to rescue them. This is the mine rescue tradition. Today’s mine rescue efforts are highly organized operations carried out by groups of trained and skilled individuals who work together as a team. Regulations require all underground mines to have fully-trained and equipped professional mine rescue teams available in the event of a mine emergency. MSHA’s Mine Rescue Instruction Guide (IG) series is intended to help your mine to meet mine rescue team training requirements under 30 CFR Part 49. The materials in this series are divided into self-contained units of study called “modules.” Each module covers a separate subject and includes suggestions, handouts, visuals, and text materials to assist you with training. Instructors and trainers may wish to use these materials to either supplement existing mine rescue training, or tailor a program to fit their mine-specific training needs.