The Mir-378C-Samd1 Circuit Promotes Phenotypic Modulation of Vascular Smooth Muscle Cells and Foam Cells Formation in Atheroscle
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Federal Register/Vol. 83, No. 221/Thursday, November 15, 2018
Federal Register / Vol. 83, No. 221 / Thursday, November 15, 2018 / Notices 57421 with regard to this program, which had Public Comment DEPARTMENT OF COMMERCE the lowest rate in the Preliminary Determination among the programs Case briefs or other written comments International Trade Administration may be submitted to the Assistant alleged to be inconsistent with the SCM [A–570–073] Agreement. In so doing, we intend to Secretary for Enforcement and limit the corresponding offset to the Compliance no later than seven days Antidumping Duty Investigation of dumping margin (if one is found) in the after the date on which the last Common Alloy Aluminum Sheet From companion antidumping duty verification report is issued in this the People’s Republic of China: investigation, which best fulfills our investigation. Rebuttal briefs, limited to Affirmative Final Determination of statutory mandate ‘‘to ensure that the issues raised in case briefs, may be Sales at Less-Than-Fair Value party does not obtain a more favorable submitted no later than five days after result by failing to cooperate than if it the deadline date for case briefs.20 AGENCY: Enforcement and Compliance, had cooperated fully,’’ 18 and induce Pursuant to 19 CFR 351.309(c)(2) and International Trade Administration, future cooperation by companies in (d)(2), parties who submit case briefs or Department of Commerce. investigations where the petitioners rebuttal briefs in this investigation are SUMMARY: The Department of Commerce allege the existence of programs encouraged to submit with each (Commerce) determines that common potentially inconsistent with the SCM argument: (1) A statement of the issue; alloy aluminum sheet (common alloy Agreement. -

Chinacoalchem
ChinaCoalChem Monthly Report Issue May. 2019 Copyright 2019 All Rights Reserved. ChinaCoalChem Issue May. 2019 Table of Contents Insight China ................................................................................................................... 4 To analyze the competitive advantages of various material routes for fuel ethanol from six dimensions .............................................................................................................. 4 Could fuel ethanol meet the demand of 10MT in 2020? 6MTA total capacity is closely promoted ....................................................................................................................... 6 Development of China's polybutene industry ............................................................... 7 Policies & Markets ......................................................................................................... 9 Comprehensive Analysis of the Latest Policy Trends in Fuel Ethanol and Ethanol Gasoline ........................................................................................................................ 9 Companies & Projects ................................................................................................... 9 Baofeng Energy Succeeded in SEC A-Stock Listing ................................................... 9 BG Ordos Started Field Construction of 4bnm3/a SNG Project ................................ 10 Datang Duolun Project Created New Monthly Methanol Output Record in Apr ........ 10 Danhua to Acquire & -

Argus China Petroleum News and Analysis on Oil Markets, Policy and Infrastructure
Argus China Petroleum News and analysis on oil markets, policy and infrastructure Volume XII, 1 | January 2018 Yuan for the road EDITORIAL: Regional gasoline The desire to avoid tax has been a far more significant factor underlying imports markets are so far unmoved by a of mixed aromatics than China’s octane deficit. potential fall in Chinese exports The government has announced plans to make it impossible to buy or sell owing to stricter tax enforcement gasoline without producing a complete invoice chain showing that consumption tax has been paid, from 1 March. And gasoline refining margins shot to nearly $20/bl, their highest since mid-2015. Of course, Beijing has tried to stamp out tax evasion in the gasoline market many times before. But, if successful, this poses Mixed aromatics imports 2017 an existential threat — to trading companies and the blending firms that use ’000 b/d Mideast mixed aromatics to produce gasoline outside the refining system, largely avoiding US Gulf 4.39 the Yn2,722/t ($51/bl) tax collected on gasoline produced by refineries. Around 22.59 300,000 b/d of gasoline is produced this way. And that has caused the surplus that forces state-owned firms to market their costlier fuel overseas. Europe But there is little panic outside south China, where most blending takes place. 77.69 The Singapore market is discounting any threat that a crackdown on tax avoidance might choke off Chinese exports — gasoline crack spreads fell this month. China’s prices are now above those in Singapore, yet its gasoline exports show no sign of letting up. -

DNA Replication and Sister Chromatid Cohesion 1 Promotes Breast Carcinoma Progression by Modulating the Wnt/B-Catenin Signaling and P53 Protein
J Biosci (2020)45:127 Ó Indian Academy of Sciences DOI: 10.1007/s12038-020-00100-y (0123456789().,-volV)(0123456789().,-volV) DNA replication and sister chromatid cohesion 1 promotes breast carcinoma progression by modulating the Wnt/b-catenin signaling and p53 protein 1 2 3 4 GUANGCHAO JIN ,WENSHENG WANG ,PENG CHENG ,YUNQI TIAN , 1 1 LUXIAO ZHANG and HU NIU * 1Department of Breast and Thyroid Surgery, Jinan Central Hospital Affiliated to Shandong First Medical University, Jinan 250013, Shandong, China 2Department of Emergency Medicine, Xintai People’s Hospital, Xintai 271200, Shandong, China 3Department of General Surgery, No.2 People’s Hospital of Xintai, Xintai 271219, Shandong, China 4Department of Gastroenterology Department 3, Jinan Central Hospital Affiliated to Shandong First Medical University, Jinan 250013, Shandong, China *Corresponding author (Email, [email protected]) MS received 30 July 2020; accepted 21 September 2020 The objective of this study is to assess the prognostic and functional role of DSCC1 in breast carcinoma, as well as the potential mechanism. Based upon the TCGA data, the expression pattern and prognostic value of DSCC1 in breast carcinoma was evaluated. The mRNA and protein levels of molecules were determined using qRT-PCR and Western blot. In vitro functional role of DSCC1 in tumor cells was determined using cell counting kit 8, clone formation, and Transwell assays. Gene set enrichment analysis (GSEA) was conducted to determine DSCC1 related gene sets, which are further confirmed by Western blot. The results showed that DSCC1 is overexpressed in breast carcinoma tissues and its high expression was linked to shorter overall survival. Overexpression of DSCC1 facilitated the proliferation, invasion and migration of breast carcinoma cells, while knockdown of DSCC1 showed opposite outcomes. -
Three Kingdoms Unveiling the Story: List of Works
Celebrating the 40th Anniversary of the Japan-China Cultural Exchange Agreement List of Works Organizers: Tokyo National Museum, Art Exhibitions China, NHK, NHK Promotions Inc., The Asahi Shimbun With the Support of: the Ministry of Foreign Affairs of Japan, NATIONAL CULTURAL HERITAGE ADMINISTRATION, July 9 – September 16, 2019 Embassy of the People’s Republic of China in Japan With the Sponsorship of: Heiseikan, Tokyo National Museum Dai Nippon Printing Co., Ltd., Notes Mitsui Sumitomo Insurance Co.,Ltd., MITSUI & CO., LTD. ・Exhibition numbers correspond to the catalogue entry numbers. However, the order of the artworks in the exhibition may not necessarily be the same. With the cooperation of: ・Designation is indicated by a symbol ☆ for Chinese First Grade Cultural Relic. IIDA CITY KAWAMOTO KIHACHIRO PUPPET MUSEUM, ・Works are on view throughout the exhibition period. KOEI TECMO GAMES CO., LTD., ・ Exhibition lineup may change as circumstances require. Missing numbers refer to works that have been pulled from the JAPAN AIRLINES, exhibition. HIKARI Production LTD. No. Designation Title Excavation year / Location or Artist, etc. Period and date of production Ownership Prologue: Legends of the Three Kingdoms Period 1 Guan Yu Ming dynasty, 15th–16th century Xinxiang Museum Zhuge Liang Emerges From the 2 Ming dynasty, 15th century Shanghai Museum Mountains to Serve 3 Narrative Figure Painting By Qiu Ying Ming dynasty, 16th century Shanghai Museum 4 Former Ode on the Red Cliffs By Zhang Ruitu Ming dynasty, dated 1626 Tianjin Museum Illustrated -

Tier 1 Manufacturing Sites
TIER 1 MANUFACTURING SITES - Produced January 2021 SUPPLIER NAME MANUFACTURING SITE NAME ADDRESS PRODUCT TYPE No of EMPLOYEES Albania Calzaturificio Maritan Spa George & Alex 4 Street Of Shijak Durres Apparel 100 - 500 Calzificio Eire Srl Italstyle Shpk Kombinati Tekstileve 5000 Berat Apparel 100 - 500 Extreme Sa Extreme Korca Bul 6 Deshmoret L7Nr 1 Korce Apparel 100 - 500 Bangladesh Acs Textiles (Bangladesh) Ltd Acs Textiles & Towel (Bangladesh) Tetlabo Ward 3 Parabo Narayangonj Rupgonj 1460 Home 1000 - PLUS Akh Eco Apparels Ltd Akh Eco Apparels Ltd 495 Balitha Shah Belishwer Dhamrai Dhaka 1800 Apparel 1000 - PLUS Albion Apparel Group Ltd Thianis Apparels Ltd Unit Fs Fb3 Road No2 Cepz Chittagong Apparel 1000 - PLUS Asmara International Ltd Artistic Design Ltd 232 233 Narasinghpur Savar Dhaka Ashulia Apparel 1000 - PLUS Asmara International Ltd Hameem - Creative Wash (Laundry) Nishat Nagar Tongi Gazipur Apparel 1000 - PLUS Aykroyd & Sons Ltd Taqwa Fabrics Ltd Kewa Boherarchala Gila Beradeed Sreepur Gazipur Apparel 500 - 1000 Bespoke By Ges Unip Lda Panasia Clothing Ltd Aziz Chowdhury Complex 2 Vogra Joydebpur Gazipur Apparel 1000 - PLUS Bm Fashions (Uk) Ltd Amantex Limited Boiragirchala Sreepur Gazipur Apparel 1000 - PLUS Bm Fashions (Uk) Ltd Asrotex Ltd Betjuri Naun Bazar Sreepur Gazipur Apparel 500 - 1000 Bm Fashions (Uk) Ltd Metro Knitting & Dyeing Mills Ltd (Factory-02) Charabag Ashulia Savar Dhaka Apparel 1000 - PLUS Bm Fashions (Uk) Ltd Tanzila Textile Ltd Baroipara Ashulia Savar Dhaka Apparel 1000 - PLUS Bm Fashions (Uk) Ltd Taqwa -

Download File
On the Periphery of a Great “Empire”: Secondary Formation of States and Their Material Basis in the Shandong Peninsula during the Late Bronze Age, ca. 1000-500 B.C.E Minna Wu Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Graduate School of Arts and Sciences COLUMIBIA UNIVERSITY 2013 @2013 Minna Wu All rights reserved ABSTRACT On the Periphery of a Great “Empire”: Secondary Formation of States and Their Material Basis in the Shandong Peninsula during the Late Bronze-Age, ca. 1000-500 B.C.E. Minna Wu The Shandong region has been of considerable interest to the study of ancient China due to its location in the eastern periphery of the central culture. For the Western Zhou state, Shandong was the “Far East” and it was a vast region of diverse landscape and complex cultural traditions during the Late Bronze-Age (1000-500 BCE). In this research, the developmental trajectories of three different types of secondary states are examined. The first type is the regional states established by the Zhou court; the second type is the indigenous Non-Zhou states with Dong Yi origins; the third type is the states that may have been formerly Shang polities and accepted Zhou rule after the Zhou conquest of Shang. On the one hand, this dissertation examines the dynamic social and cultural process in the eastern periphery in relation to the expansion and colonization of the Western Zhou state; on the other hand, it emphasizes the agency of the periphery during the formation of secondary states by examining how the polities in the periphery responded to the advances of the Western Zhou state and how local traditions impacted the composition of the local material assemblage which lay the foundation for the future prosperity of the regional culture. -

World Bank Document
The World Bank Report No: ISR15381 Implementation Status & Results China Shandong Ecological Afforestation (P112759) Operation Name: Shandong Ecological Afforestation (P112759) Project Stage: Implementation Seq.No: 6 Status: ARCHIVED Archive Date: 24-Jun-2014 Country: China Approval FY: 2010 Public Disclosure Authorized Product Line:IBRD/IDA Region: EAST ASIA AND PACIFIC Lending Instrument: Specific Investment Loan Implementing Agency(ies): Shandong Provincial Forestry Department Key Dates Board Approval Date 06-May-2010 Original Closing Date 31-Jul-2016 Planned Mid Term Review Date Last Archived ISR Date 17-Dec-2013 Public Disclosure Copy Effectiveness Date 11-Aug-2010 Revised Closing Date 31-Jul-2016 Actual Mid Term Review Date Project Development Objectives Project Development Objective (from Project Appraisal Document) The Project Development Objective is to demonstrate effective afforestation models for environmentally degraded areas in support ofShandong's environmental afforestation programs. Has the Project Development Objective been changed since Board Approval of the Project? Yes No Public Disclosure Authorized Component(s) Component Name Component Cost Environmental Plantation Establishment 101.95 Technical Support and Project Management 6.35 Overall Ratings Previous Rating Current Rating Progress towards achievement of PDO Satisfactory Satisfactory Overall Implementation Progress (IP) Satisfactory Satisfactory Overall Risk Rating Moderate Moderate Public Disclosure Authorized Implementation Status Overview The project has made very good progress over the past years. Both project physical progress and IBRD Loan disbursement are ahead of schedule. The project agencies have been successful in promoting the implementation of innovative ecological afforestation models to restore forest cover in highly degraded mountainous areas and saline coastal land to reduce water, soil and wind erosions as well as to stabilize newly created alluvial lands. -
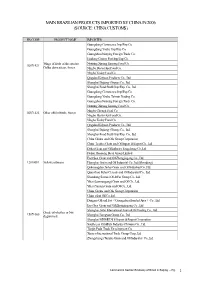
Main Brazilian Products Imported by China in 2006 (Source: China Customs)
MAIN BRAZILIAN PRODUCTS IMPORTED BY CHINA IN 2006 (SOURCE: CHINA CUSTOMS) HS CODE PRODUCT NAME IMPORTER Guangdong Commence Imp/Exp Co. Guangdong Youhe Imp/Exp Co. Guangzhou Nanying Foreign Trade Co. Huidong County Port Imp/Exp Co. Wings of fowls of the species Nanning Xinxing Kenong Food Co. 02071421 Gallus domesticus, frozen Ningbo Doctor Kai Food Co. Ningbo Today Food Co. Qingdao Kaiyuan Products Co., Ltd. Shanghai Dajiang (Group) Co., Ltd. Shanghai Food Stuffs Imp/Exp. Co., Ltd. Guangdong Commence Imp/Exp Co. Guangdong Youhe Taiwan Trading Co. Guangzhou Nanying Foreign Trade Co. Nanning Xinxing Kenong Food Co. Ningbo Chengji Food Co. 02071422 Other offal of fowls, frozen Ningbo Doctor Kai Food Co. Ningbo Today Food Co. Qingdao Kaiyuan Products Co., Ltd. Shanghai Dajiang (Group) Co., Ltd. Shanghai Food Stuffs Imp/Exp. Co., Ltd. China Grains and Oils Group Corporation China Textiles Grain and Oil Import & Export Co., Ltd. Dahai Grain and Oil Industry Fangcheng Co.,Ltd. Dalian Huanong Bean Group Limited East Sea Grain and Oil Zhangjiagang Co., Ltd 12010091 Yellow soybeans Huanghai Grain and Oil Industrial Co.,Ltd.(Shandong) Qinhuangdao Jinhai Grain and Oil Industrial Co.,Ltd. Quanzhou Fuhai Cereals and Oil Industrial Co., Ltd. Shandong Sanwei Oil & Fat Group Co., Ltd. Yihai (Lianyungang) Grain and Oil Co., Ltd. Yihai (Yantai) Grain and Oil Co., Ltd. China Grains and Oils Group Corporation China plant Oil Co.,Ltd. Dongma Oil and Fat(Guangzhou Bonded Area)Co., Ltd. East Sea Grain and Oil Zhangjiagang Co., Ltd Shanghai Jintai International Grain & Oil Trading Co., Ltd. Crude oil whether or Not 15071000 degummed Shanghai Liangyou Group Co., Ltd. -

Targeted Inhibition of the Phosphoinositide 3-Kinase Impairs Cell Proliferation, Survival, and Invasion in Colon Cancer
Journal name: OncoTargets and Therapy Article Designation: Original Research Year: 2017 Volume: 10 OncoTargets and Therapy Dovepress Running head verso: Yang et al Running head recto: Phosphoinositide 3-kinase in colon cancer open access to scientific and medical research DOI: http://dx.doi.org/10.2147/OTT.S145601 Open Access Full Text Article ORIGINAL RESEARCH Targeted inhibition of the phosphoinositide 3-kinase impairs cell proliferation, survival, and invasion in colon cancer Fei Yang1,* Background: Colon cancer is the third most common cancer in the world, and its metastasis Jun-Yi Gao2,* and drug resistance are challenging for its effective treatment. The PI3K/Akt/mTOR pathway Hua Chen1 plays a crucial role in the pathogenesis of colon cancer. The aim of this study was to investigate Zhen-Hua Du1 the targeting of PI3K in colon cancer cells HT-29 and HCT-116 in vitro. Xue-Qun Zhang3 Methods: In HT-29 and HCT-116 cells, BEZ235, a dual inhibitor of PI3K/mTOR, and Wei Gao4 shRNAtarget to PI3KCA were used to inhibit PI3K/Akt/mTOR pathway. The inhibition effi- ciency of PI3K/Akt/mTOR pathway was detected by RT-PCR and Western blot. Cell prolifera- 1 Department of Pathology, tion, migration, invasion, and apoptosis were evaluated by Cell Counting Kit-8, Transwell, and Jinan Central Hospital Affiliated to Shandong University, Jinan, flow cytometry assays. The expression of apoptosis-related proteins (cleavage caspase 3, Bcl-2, 2 For personal use only. Department of Clinical Medicine, Bax, and Bim) were also detected. Weifang Medical College, Weifang, We found that in HT-29 and HCT-116 cells, the treatment of BEZ235 (1 M) and 3Graduate School, Taishan Medical Results: µ University, Xintai, 4Department of PI3KCA knockdown inhibited the activation of PI3K/Akt/mTOR pathway and significantly Oncology, Jinan Central Hospital suppressed cell proliferation, migration, and invasion of HT-29 and HCT-116 cells. -

For Personal Use Only Use Personal For
13 July 2012 Norton Rose Australia ABN 32 720 868 049 By e-Lodgement Level 15, RACV Tower 485 Bourke Street Company Announcements MELBOURNE VIC 3000 Australian Securities Exchange Limited AUSTRALIA Level 2 120 King Street Tel +61 3 8686 6000 MELBOURNE VIC 3000 Fax +61 3 8686 6505 GPO Box 4592, Melbourne VIC 3001 DX 445 Melbourne nortonrose.com Direct line +61 3 8686 6710 Our reference Email 2781952 [email protected] Dear Sir/Madam Form 604 – Notice of change of interests of substantial shareholder We act for Linyi Mining Group Co., Ltd. (Linyi), a wholly owned subsidiary of Shandong Energy Group Co., Ltd. (Shandong Energy), in relation to its off-market takeover bid for all of the ordinary shares in Rocklands Richfield Limited ABN 82 057 121 749 (RCI) (Offer) on the terms and conditions set out in Linyi’s bidder’s statement dated 7 June 2012. On behalf of Linyi, Shandong Energy and their related bodies corporate, we enclose a notice of change of interests of substantial shareholder (Form 604) in respect of RCI. A copy of the enclosed Form 604 is being provided to RCI today. Yours faithfully James Stewart Partner Norton Rose Australia Encl. For personal use only APAC-#15206384-v1 Norton Rose Australia is a law firm as defined in the Legal Profession Acts of the Australian states and territory in which it practises. Norton Rose Australia together with Norton Rose LLP, Norton Rose Canada LLP, Norton Rose South Africa (incorporated as Deneys Reitz Inc) and their respective affiliates constitute Norton Rose Group, an international legal practice with offices worldwide, details of which, with certain regulatory information, are at nortonrose.com 604 page 2/2 15 July 2001 Form 604 Corporations Act 2001 Section 671B Notice of change of interests of substantial holder To Company Name/Scheme Rocklands Richfield Limited (RCI) ACN/ARSN ACN 057 121 749 1. -

A Crazy Gamble: Xintai Electric in China Malicious Fabrication and Packaging Market Case
American Journal of Industrial and Business Management, 2018, 8, 393-403 http://www.scirp.org/journal/ajibm ISSN Online: 2164-5175 ISSN Print: 2164-5167 A Crazy Gamble: Xintai Electric in China Malicious Fabrication and Packaging Market Case Wenjing Ma Accounting Department, Management School, Jinan University, Guangzhou, China How to cite this paper: Ma, W.J. (2018) A Abstract Crazy Gamble: Xintai Electric in China Malicious Fabrication and Packaging Mar- On June 1st, 2016, Xintai Electric Co., Ltd. released an announcement that ket Case. American Journal of Industrial China Securities Regulatory Commission (CSCR) had finished its investiga- and Business Management, 8, 393-403. tion for the company’s suspicion of fraudulent issuing and illegal information https://doi.org/10.4236/ajibm.2018.82025 disclosure, according to the administrative penalty and market access prohibi- Received: January 18, 2018 tion notification from CSCR. Xintai became the first company to be delisted Accepted: February 24, 2018 due to fraudulent issuing, which drew concerns from numerous investors and Published: February 27, 2018 regulators. August 25th, 2017 was the last day Xintai occupied an A-share Copyright © 2018 by author and market. From listing to delisting, going through three and a half years, Xintai Scientific Research Publishing Inc. left the market with a serious warning that the first company to be forcibly de- This work is licensed under the Creative listed in growth enterprise market for fraudulent initial public offering (IPO) Commons Attribution International will never appear on the market again. The present article conducts research License (CC BY 4.0). http://creativecommons.org/licenses/by/4.0/ into the internal control structure of listed company, in view of Xintai’s finan- Open Access cial fraud case.