Решение Комиссии Таможенного Союза От 28 Мая 2010 Года №299 «О Применении Санитарных Мер В Таможенном Союзе»
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mt Handbook 0.Pdf
TABLE OF CONTENTS Page No. Chapter 1: Introduction 1.1. Status, market size and scope of fruit and vegetable processing 4 industry in India 1.2. Selection and procurement 5 1.3. Supply chain of fruits and vegetables 15 1.4. Pack house handling of fruits and vegetables 19 1.5. Primary Processing of Fruits and Vegetables 26 1.6. Unit operations in fruits and vegetables processing 30 1.7. Minimal Processing of Fruits and Vegetables 42 Chapter 2: Value addition of Fruits and Vegetables 2.1. Processing of fruit pulp/puree 49 2.2. Processing of Jams 50 2.3. Processing of Jellies 54 2.4. Processing of osmotic dehydrated product 59 2.5. Preservation by chemicals 63 2.6. Processing of Sauce 65 2.7. Processing of Ketchup 67 2.8. Processing of Pickles 73 2.9. Processing of Chutneys 80 2.10. Processing of Fruit Juices 82 Chapter 3: Packaging of Fruits and Vegetable Products 3.1. Levels, functions and desirable features of packaging 93 3.2. Packaging materials and their properties 95 3.3. Special Packaging systems 112 3.4. Properties/characteristics of foods, packaging requirement and 115 methods 3.5. MAP of fruits and vegetables 116 3.6. Mechanical injuries of fruits and vegetables 118 3.7. Shelf life of packaged food and its determination 121 3.8. Storage of Fruits and Vegetables 124 Chapter 4: Food safety regulations & certification 4.1. Need for testing of food 130 4.2. List of Notified Reference Laboratories in India – 1 131 4.3. List of Notified Reference Laboratories in India – 2 132 4.4. -

Mineral Processing
Mineral Processing Foundations of theory and practice of minerallurgy 1st English edition JAN DRZYMALA, C. Eng., Ph.D., D.Sc. Member of the Polish Mineral Processing Society Wroclaw University of Technology 2007 Translation: J. Drzymala, A. Swatek Reviewer: A. Luszczkiewicz Published as supplied by the author ©Copyright by Jan Drzymala, Wroclaw 2007 Computer typesetting: Danuta Szyszka Cover design: Danuta Szyszka Cover photo: Sebastian Bożek Oficyna Wydawnicza Politechniki Wrocławskiej Wybrzeze Wyspianskiego 27 50-370 Wroclaw Any part of this publication can be used in any form by any means provided that the usage is acknowledged by the citation: Drzymala, J., Mineral Processing, Foundations of theory and practice of minerallurgy, Oficyna Wydawnicza PWr., 2007, www.ig.pwr.wroc.pl/minproc ISBN 978-83-7493-362-9 Contents Introduction ....................................................................................................................9 Part I Introduction to mineral processing .....................................................................13 1. From the Big Bang to mineral processing................................................................14 1.1. The formation of matter ...................................................................................14 1.2. Elementary particles.........................................................................................16 1.3. Molecules .........................................................................................................18 1.4. Solids................................................................................................................19 -

No 1333/2008 of the EUROPEAN PARLIAMENT and of the COUNCIL of 16 December 2008 on Food Additives (Text with EEA Relevance)
2008R1333 — EN — 21.04.2015 — 022.001 — 1 This document is meant purely as a documentation tool and the institutions do not assume any liability for its contents ►B REGULATION (EC) No 1333/2008 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 16 December 2008 on food additives (Text with EEA relevance) (OJ L 354, 31.12.2008, p. 16) Amended by: Official Journal No page date ►M1 Commission Regulation (EU) No 238/2010 of 22 March 2010 L 75 17 23.3.2010 ►M2 Commission Regulation (EU) No 1129/2011 of 11 November 2011 L 295 1 12.11.2011 ►M3 amended by Commission Regulation (EU) No 1152/2013 of 19 L 311 1 20.11.2013 November 2013 ►M4 Commission Regulation (EU) No 1130/2011 of 11 November 2011 L 295 178 12.11.2011 ►M5 Commission Regulation (EU) No 1131/2011 of 11 November 2011 L 295 205 12.11.2011 ►M6 Commission Regulation (EU) No 232/2012 of 16 March 2012 L 78 1 17.3.2012 ►M7 Commission Regulation (EU) No 380/2012 of 3 May 2012 L 119 14 4.5.2012 ►M8 Commission Regulation (EU) No 470/2012 of 4 June 2012 L 144 16 5.6.2012 ►M9 Commission Regulation (EU) No 471/2012 of 4 June 2012 L 144 19 5.6.2012 ►M10 Commission Regulation (EU) No 472/2012 of 4 June 2012 L 144 22 5.6.2012 ►M11 Commission Regulation (EU) No 570/2012 of 28 June 2012 L 169 43 29.6.2012 ►M12 Commission Regulation (EU) No 583/2012 of 2 July 2012 L 173 8 3.7.2012 ►M13 Commission Regulation (EU) No 675/2012 of 23 July 2012 L 196 52 24.7.2012 ►M14 Commission Regulation (EU) No 1049/2012 of 8 November 2012 L 310 41 9.11.2012 ►M15 Commission Regulation (EU) No 1057/2012 of 12 November 2012 -

Tanibirumab (CUI C3490677) Add to Cart
5/17/2018 NCI Metathesaurus Contains Exact Match Begins With Name Code Property Relationship Source ALL Advanced Search NCIm Version: 201706 Version 2.8 (using LexEVS 6.5) Home | NCIt Hierarchy | Sources | Help Suggest changes to this concept Tanibirumab (CUI C3490677) Add to Cart Table of Contents Terms & Properties Synonym Details Relationships By Source Terms & Properties Concept Unique Identifier (CUI): C3490677 NCI Thesaurus Code: C102877 (see NCI Thesaurus info) Semantic Type: Immunologic Factor Semantic Type: Amino Acid, Peptide, or Protein Semantic Type: Pharmacologic Substance NCIt Definition: A fully human monoclonal antibody targeting the vascular endothelial growth factor receptor 2 (VEGFR2), with potential antiangiogenic activity. Upon administration, tanibirumab specifically binds to VEGFR2, thereby preventing the binding of its ligand VEGF. This may result in the inhibition of tumor angiogenesis and a decrease in tumor nutrient supply. VEGFR2 is a pro-angiogenic growth factor receptor tyrosine kinase expressed by endothelial cells, while VEGF is overexpressed in many tumors and is correlated to tumor progression. PDQ Definition: A fully human monoclonal antibody targeting the vascular endothelial growth factor receptor 2 (VEGFR2), with potential antiangiogenic activity. Upon administration, tanibirumab specifically binds to VEGFR2, thereby preventing the binding of its ligand VEGF. This may result in the inhibition of tumor angiogenesis and a decrease in tumor nutrient supply. VEGFR2 is a pro-angiogenic growth factor receptor -

1 Abietic Acid R Abrasive Silica for Polishing DR Acenaphthene M (LC
1 abietic acid R abrasive silica for polishing DR acenaphthene M (LC) acenaphthene quinone R acenaphthylene R acetal (see 1,1-diethoxyethane) acetaldehyde M (FC) acetaldehyde-d (CH3CDO) R acetaldehyde dimethyl acetal CH acetaldoxime R acetamide M (LC) acetamidinium chloride R acetamidoacrylic acid 2- NB acetamidobenzaldehyde p- R acetamidobenzenesulfonyl chloride 4- R acetamidodeoxythioglucopyranose triacetate 2- -2- -1- -β-D- 3,4,6- AB acetamidomethylthiazole 2- -4- PB acetanilide M (LC) acetazolamide R acetdimethylamide see dimethylacetamide, N,N- acethydrazide R acetic acid M (solv) acetic anhydride M (FC) acetmethylamide see methylacetamide, N- acetoacetamide R acetoacetanilide R acetoacetic acid, lithium salt R acetobromoglucose -α-D- NB acetohydroxamic acid R acetoin R acetol (hydroxyacetone) R acetonaphthalide (α)R acetone M (solv) acetone ,A.R. M (solv) acetone-d6 RM acetone cyanohydrin R acetonedicarboxylic acid ,dimethyl ester R acetonedicarboxylic acid -1,3- R acetone dimethyl acetal see dimethoxypropane 2,2- acetonitrile M (solv) acetonitrile-d3 RM acetonylacetone see hexanedione 2,5- acetonylbenzylhydroxycoumarin (3-(α- -4- R acetophenone M (LC) acetophenone oxime R acetophenone trimethylsilyl enol ether see phenyltrimethylsilyl... acetoxyacetone (oxopropyl acetate 2-) R acetoxybenzoic acid 4- DS acetoxynaphthoic acid 6- -2- R 2 acetylacetaldehyde dimethylacetal R acetylacetone (pentanedione -2,4-) M (C) acetylbenzonitrile p- R acetylbiphenyl 4- see phenylacetophenone, p- acetyl bromide M (FC) acetylbromothiophene 2- -5- -

Pvc Piping Systems for Commercial and Industrial Applications
PVC PIPING SYSTEMS FOR COMMERCIAL AND INDUSTRIAL APPLICATIONS Plastic Pipe and Fittings Association © 2012 Plastic Pipe and Fittings Association (PPFA) Acknowledgments We would like to thank the following contributors to the Design Guide: The PVC and Thermoplastic Industrial Piping Systems (TIPS) Product Line Committees and member companies of the Plastic Pipe and Fittings Association (PPFA). In particular the following PPFA companies and individuals ably assisted in reviewing the text and tables and provided valuable comments which added greatly in producing a better and more accurate source document: Chuck Bush – Oatey Company Mike Cudahy – PPFA Staff Patrick Fedor – IPEX Bill Morris – Charlotte Pipe & Foundry Jack Roach – Mueller Industries Bill Weaver – Harvel Plastics Larry Workman – LASCO Fittings All text, tables and photos were prepared and or edited by David A. Chasis of Chasis Consulting, Inc. Using the Design Guide The Design Guide was created to assist engineers, installers, end-users, engineering students and building code officials in learning more of the dos and don’ts of PVC piping systems. The Design Guide is comprised of ten sections including: Introduction Features and Benefits Engineering Design Joining Methods Installation Testing and Repair Applications Building Codes, Standards, and Sample Specifications PVC Piping and the Environment Other Plastic Piping Systems In addition, in the back of the guide is the most complete appendix and glossary of PVC piping systems ever assembled. Other PPFA Educational Materials The PPFA offers a wide range of other educational materials developed to assist the engineering and construction industry to become more proficient in the use of the preferred piping system...plastics! On-site seminars, Webinars, CD-based seminars, workbooks, online tutorials and product and technical literature are available. -
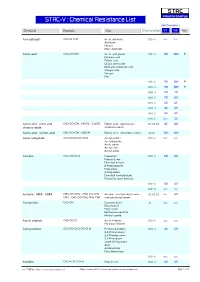
STAC-V : Chemical Resistance List Max Temperature
S TA C Industrial Coatings STAC-V : Chemical Resistance List Max Temperature Chemical Formula Alias Concentration V1 V2 Note Acetaldehyde CH3-CH=O Acetic aldehyde 100 % n.r. n.r. Aldehyde Ethanal Ethyl aldehyde Acetic acid CH3-CO-OH Acetic acid glacial 010 % 90 100 0 Ethanoic acid Ethylic acid Glacial acetic acid Methane carboxylic acid Vinegar acid Vinegar Hac 015 % 90 100 0 025 % 90 100 0 040 % 80 90 050 % 70 80 075 % 60 65 080 % 45 45 085 % 45 45 100 % n.r. 25 Acetic acid : nitric acid : CH3-CO-OH : HNO3 : Cr2O3 Ethylic acid : salpeterzuur : 03:05:03 65 80 chromic oxide chromium oxide Acetic acid : sulfuric acid CH3-CO-OH : H2SO4 Ethylic acid : dihydrogen sulfate 20:10 100 100 Acetic anhydride CH3-CO-O-CO-CH3 Acetyl acetate 100 % n.r. n.r. Acetanhydride Acetic oxide Acetyl ether Acetyl oxide Acetone CH3-CO-CH3 Propanone 005 % 80 80 Propan-2-one Dimethyl ketone β-Ketopropane[ Propanone 2-Propanone Dimethyl formaldehyde Pyroacetic spirit (archaic) 010 % 80 80 100 % n.r. n.r. Acetone : MEK : MiBK CH3-CO-CH3 : CH3-CO-CH2- Acetone : methylethyl ketone : 02:02:02 n.r. 40 CH3 : CH3-CO-CH2-CH2-CH3 methylisobutyl ketone Acetonitrile CH3-CN Cyanomethane all n.r. n.r. Ethanenitrile Ethyl nitrile Methanecarbonitrile Methyl cyanid Acetyl chloride CH3-CO-Cl Acetic chloride 100 % n.r. n.r. Ethanoyl chloride Acetylacetone CH3-CO-CH2-CO-CH3 Pentane-2,4-dione 020 % 40 50 2,4-Pentanedione 2,4-Dioxopentane 2,4-Pentadione acetyl-2-Propanone Acac Acetoacetone Diacetylmethane 100 % n.r. -

Chapter 1: Introduction
ZAZ 0701 Finding Green Solutions for Nypro, Inc A Major Qualifying Project Submitted to the Faculty Of the WORCESTER POLYTECHNIC INSTITUTE In Partial Fulfillment of the requirements for the Degree of Bachelor of Science By ________________________ Emily Allietta ________________________ Catherine Maki Date: March 4, 2008 Approved: __________________________________ Professor Amy Zeng, Primary Advisor 1 Table of Contents Table of Contents ................................................................................................................ 2 Table of Figures .................................................................................................................. 4 Abstract ............................................................................................................................... 5 Acknowledgements ............................................................................................................. 6 1 Introduction ...................................................................................................................... 7 2 Literature Review........................................................................................................... 10 2.1 Purchasing Strategies .............................................................................................. 10 2.2 Energy and Gas ....................................................................................................... 11 2.2.1 Gas Trends ...................................................................................................... -

Whole Foods Market Unacceptable Ingredients for Food (As of March 15, 2019)
Whole Foods Market Unacceptable Ingredients for Food (as of March 15, 2019) 2,4,5-trihydroxybutyrophenone (THBP) benzoyl peroxide acesulfame-K benzyl alcohol acetoin (synthetic) beta-cyclodextrin acetone peroxides BHA (butylated hydroxyanisole) acetylated esters of mono- and diglycerides BHT (butylated hydroxytoluene) activated charcoal bleached flour advantame bromated flour aluminum ammonium sulfate brominated vegetable oil aluminum potassium sulfate burnt alum aluminum starch octenylsuccinate butylparaben aluminum sulfate caffeine (extended release) ammonium alum calcium benzoate ammonium chloride calcium bromate ammonium saccharin calcium disodium EDTA ammonium sulfate calcium peroxide apricot kernel/extract calcium propionate artificial sweeteners calcium saccharin aspartame calcium sorbate azo dyes calcium stearoyl-2-lactylate azodicarbonamide canthaxanthin bacillus subtilis DE111 caprocaprylobehenin bacteriophage preparation carmine bentonite CBD/cannabidiol benzoates certified colors benzoic acid charcoal powder benzophenone Citrus Red No. 2 Page 1 of 4 cochineal foie gras DATEM gardenia blue diacetyl (synthetic) GMP dimethyl Silicone gold/gold leaf dimethylpolysiloxane heptylparaben dioctyl sodium sulfosuccinate (DSS) hexa-, hepta- and octa-esters of sucrose disodium 5'-ribonucleotides high-fructose corn syrup/HFCS disodium calcium EDTA hjijiki disodium dihydrogen EDTA hydrogenated oils disodium EDTA inosine monophosphate disodium guanylate insect Flour disodium inosinate iron oxide dodecyl gallate kava/kava kava EDTA lactic acid esters of monoglycerides erythrosine lactylated esters of mono- and diglycerides ethoxyquin ma huang ethyl acrylate (synthetic) methyl silicon ethyl vanillin (synthetic) methylparaben ethylene glycol microparticularized whey protein derived fat substitute ethylene oxide monoammonium glutamate eugenyl methyl ether (synthetic) monopotassium glutamate FD&C Blue No. 1 monosodium glutamate FD&C Blue No. 2 myrcene (synthetic) FD&C Colors natamycin (okay in cheese-rind wax) FD&C Green No. -

RANGE GUIDE Johnlewis.Com Kitchen KBRG/08.18
Shop locations Autumn 2018 LONDON John Lewis Cribbs Causeway John Lewis Sheffield The Mall at Cribbs Causeway Barkers Pool John Lewis Bristol BS34 5QU Sheffield S1 1EP Oxford Street 0117 959 1100 0114 276 8511 London W1A 1EX 020 7629 7711 John Lewis High Wycombe John Lewis Solihull Holmers Farm Way Touchwood Peter Jones Cressex Solihull Sloane Square High Wycombe HP12 4NW West Midlands B91 3RA London SW1W 8EL 01494 462 666 0121 704 1121 020 7730 3434 John Lewis Leeds John Lewis Southampton John Lewis Brent Cross Victoria Gate West Quay Brent Cross Shopping Centre Harewood Street Southampton SO15 1QA London NW4 3FL Leeds S2 7AR 023 8021 6400 020 8202 6535 0113 394 6200 John Lewis Watford John Lewis Kingston John Lewis Leicester High Street Wood Street 2 Bath House Lane Watford WD17 2TW Kingston upon Thames Highcross Shopping Centre 01923 244 266 KT1 1TE Leicester LE1 4SA 020 8547 3000 0116 242 5777 John Lewis Welwyn Bridge Road John Lewis Stratford John Lewis Liverpool Welwyn Garden City 101 The Arcade 70 South John Street AL8 6TP Westfield Stratford City Liverpool One 01707 323 456 Montfichet Road Liverpool L1 8BJ London E20 1EL 0151 709 7070 John Lewis York 020 8532 3500 Unit C John Lewis Milton Keynes Vangarde Way John Lewis White City Central Milton Keynes York YO32 9AE Westfield London MK9 3EP 01904 557 950 Shopping Centre 01908 679 171 Ariel Way SCOTLAND London W12 7FU John Lewis Newcastle 020 8222 6400 Eldon Square John Lewis Aberdeen Newcastle upon Tyne George Street ENGLAND NE99 1AB Aberdeen AB25 1BW 0191 232 5000 01224 625 000 -

Extensions of Remarks. Hon. Donald M. Fraser
February 19, 1969 EXTENSIONS OF REMARKS 4031 EXTENSIONS OF REMARKS. AID FOR BIAFRAN CHILDREN be known as the "Ravensbrueck Lapins" tions. On the basis of his first-hand obser was of a dual nature. One aspect was to bring vations, Mr. Cohen spoke of growing problems them to the United States for medical and confronting evacuation of children by air. HON. DONALD M. FRASER surgical care. The other aspect was to obtain He brought U3 together with Mr. G. A. On Oi' MINNESOTA from the German government at Bonn ade yegbula, Permanent Secretary of Biafra, who quate compensation that would enable them had just arrived in New York on a brief gov IN THE HOUSE OP REPRESENTATIVES to live Without continued and excessive hard ernment mission. Mr. Onyegbula spoke of the Tuesday, February 18, 1969 ship. Both these parts of the project were severity of Biafra's needs. Two thousand carried out. children and 4,000 adults were dying daily Mr. FRASER. Mr. Speaker, one of the The editors now invite the readers of SR of starvation. Food and medical supplies most remarkable humanitarian efforts to join them in a fourth project. It is called were being flown into Bia.fra In larger quan directed at relieving the misery of the ABc-Aid for Biafran Children. HereWith, tities than had been possible for some Nigerian-Biafran tragedy is known as some background. months. But the situation continued to be Aid for Biafran Children-ABC. Last September, when the food blockade of critical and was apt to remain that way Biafra was at its worst, and when thousands until there was a dramatic breakthrough in One of the principals in this effort is of children were dying from protein shortage, direct access. -

Low Acyl Gellan Gum for Inclusion on the National List of Substances Allowed in Organic Production and Handling (7 CFR 205.605 (B)
Petition for Evaluation of Low Acyl Gellan Gum for Inclusion on the National List of Substances Allowed in Organic Production and Handling (7 CFR 205.605 (b) Submitted by: CP Kelco U.S., Inc. 3100 Cumberland Blvd., Suite 600 Atlanta, GA 30339 Date: 08 August 2019 CP Kelco U.S., Inc. 08 August 2019 National Organic List Petiion Low Acyl Gellan Gum Table of Contents Item A.1 — Section of National List ........................................................................................................... 4 Item A.2 — OFPA Category - Crop and Livestock Materials .................................................................... 4 Item A.3 — Inert Ingredients ....................................................................................................................... 4 1. Substance Name ................................................................................................................................... 5 2. Petitioner and Manufacturer Information ............................................................................................. 5 2.1. Corporate Headquarters ................................................................................................................5 2.2. Manufacturing/Processing Facility ...............................................................................................5 2.3. Contact for USDA Correspondence .............................................................................................5 3. Intended or Current Use .......................................................................................................................5