Rheology of Redcurrant Juices
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Raby Redcurrant Crumble Tray Bake
Raby Redcurrant Crumble tray bake Preparation total time 1 hour Makes 12 portion Nutrition: 100g per serving Kcal Fat Saturates Carbs Sugars Fibre Protein Salt 334 9.41g 5.88g 60.68g 34.92g 1.17g 3.07g 0.40g A layered traybake inspired by German Coffee shops. Ingredients For the berry filling • 200gr Raby red currants • 50g Caster Sugar • 2 tbsp Corn flour For the Cake • 220g Plain Flour • 4g Baking Powder • 2g Cinnamon • 50g Ground Almonds • 200g Caster Sugar • 80g Butter, Melted • 2 Free- range eggs • 40g Yoghurt- thinned with 2tbs milk Method 1. Preheat the oven to 180C/350F/Gas 4. Grease and line a rectangular baking tin (approximately 26cm x 20cm/10½in x 8in). 2. In a saucepan combine the fruit with 50ml/2fl oz water. Bring the fruit just to the boil, reduce the heat and simmer for 2-3 minutes. 3. In a mixing bowl combine the caster sugar with the cornflour. Stir into the fruit and continue to cook for 2-3 minutes, stirring frequently, until the mixture is thick and jammy. Set aside to cool. 4. For the crumble topping, add the flour to a mixing bowl and rub in the butter until the mixture resembles fine breadcrumbs and no large lumps of butter are left. Stir in the sugar and set aside. 5. For the cake, in a large mixing bowl, sift together the flour, baking powder and cinnamon. Stir in the ground almonds until thoroughly combined. 6. In another bowl beat together the sugar with the melted butter, eggs, yoghurt and milk mixture until thoroughly mixed. -

Provision of Information on the Amount of Sugar in Processed Foods
DOI: 10.1590/1413-81232021263.07872019 1153 Provision of information on the amount of sugar FREE THEMES in processed foods Camila Cremonezi Japur (https://orcid.org/0000-0003-0513-1758) 1,4 Dyessa Cardoso Bernardes Assunção (https://orcid.org/0000-0003-4711-7508) 2 Raíssa Aparecida Borges Batista (https://orcid.org/0000-0002-9928-5872) 2 Fernanda Rodrigues de Oliveira Penaforte (https://orcid.org/0000-0001-8483-1562) 3,4 Abstract The objective of this study was to assess the provision of information on the amount of sugar and identify the position of sugar in the list of ingredients of processed foods. A cross-sectional study was conducted to analyze all processed tra- ditional and diet/light/zero food products sold in a hypermarket containing the word sugar or sucrose in the list of ingredients. The food labels were read and the position of sugar on the list of ingredients and presence, or absence, of information on the amount of sugar in the nutrition facts table were 1 Divisão de Nutrição recorded. Information on the amount of sugar was e Metabolismo, Departamento de Ciências also requested from the manufacturers by e-mail da Saúde, Faculdade de or telephone. A total of 2,200 food products were Medicina de Ribeirão Preto, assessed, 2,164 (98.4%) of which were tradition- Universidade de São Paulo (USP). Av. Bandeirantes al foods and 36 (1.6%) diet/light/zero foods. The 3900, Monte Alegre. 14049- amount of sugar was declared in only 14.4% and 900 Ribeirão Preto SP 13.9% of these products, respectively (p=0.84). -
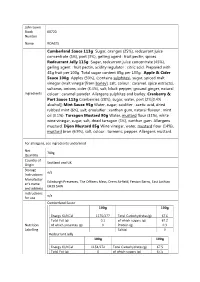
Cumberland Sauce 113G Sugar, Oranges (25%), Redcurrant Juice Concentrate (5%), Port (3%), Gelling Agent : Fruit Pectin, Spices
John Lewis Stock 60720 Number Name ROASTS Cumberland Sauce 113g Sugar, oranges (25%), redcurrant juice concentrate (5%), port (3%), gelling agent : fruit pectin, spices. Redcurrant Jelly 113g Sugar, redcurrant juice concentrate (45%), gelling agent : fruit pectin, acidity regulator : citric acid. Prepared with 45g fruit per 100g. Total sugar content 65g per 100g. Apple & Cider Sauce 100g Apples (50%), (contains sulphites), sugar, spiced malt vinegar (malt vinegar[from barley], salt, colour : caramel, spice extracts), sultanas, onions, cider (3.4%), salt, black pepper, ground ginger, natural Ingredients colour : caramel powder. Allergens sulphites and barley. Cranberry & Port Sauce 113g Cranberries (38%), sugar, water, port (2%[0.4% alcohol]) Mint Sauce 95g Water, sugar, acidifier : acetic acid, dried rubbed mint (6%), salt, emulsifier : xanthan gum, natural flavour : mint oil (0.1%). Tarragon Mustard 90g Water, mustard flour (31%), white wine vinegar, sugar, salt, dried tarragon (3%), xanthan gum. Allergens mustard. Dijon Mustard 85g Wine vinegar, water, mustard flour (14%), mustard bran (6.9%), salt, colour : turmeric, pepper. Allergens mustard. For allergens, see ingredients underlined Net 709g Quantity Country of Scotland and UK Origin Storage n/a Instructions Manufactur Edinburgh Preserves, The Officers Mess, Drem Airfield, Fenton Barns, East Lothian er’s name EH39 5AW and address Instructions n/a for use Cumberland Sauce 100g 100g Energy KJ/KCal 1175/277 Total Carbohydrates(g) 67.6 Total Fat (g) 0.1 of which sugars (g) 67.2 Nutrition -

WSET Level 2 Systematic Approach to Tasting Wine®
Level 2 SAT (A5) 2012 (v2)_Layout 1 30/03/2012 15:47 Page 1 WSET Level 2 Systematic Approach to Tasting Wine ® APPEARANCE Clarity clear – hazy Intensity pale – medium – deep Colour white lemon – gold – amber rosé pink – salmon – orange red purple – ruby – garnet – tawny NOSE Condition clean – unclean Intensity light – medium – pronounced Aroma characteristics e.g. fruits, flowers, spices, vegetables, oak aromas, other PALATE Sweetness dry – off-dry – medium – sweet Acidity low – medium – high Tannin low – medium – high Body light – medium – full Flavour characteristics e.g. fruits, flowers, spices, vegetables, oak flavours, other Finish short – medium – long CONCLUSIONS Quality faulty – poor – acceptable – good – very good – outstanding Copyright Wine & Spirit Education Trust 2012. The WSET Level 2 Systematic Approach to Tasting Wine ® may only be reproduced with the written permission of the WSET subject to their terms and conditions. For more information contact [email protected] 2W-Version 1.0 Level 2 SAT (A5) 2012 (v2)_Layout 1 30/03/2012 15:47 Page 2 WSET Level 2 Wine-Lexicon: supporting the WSET Level 2 Systematic Approach to Tasting Wine ® AROMA AND FLAVOUR CHARACTERISTICS FLORAL / FRUIT Are the flavours simple/generic or specific? Fresh or cooked? Ripe or unripe? Floral blossom, rose, violet Green Fruit green apple, red apple, gooseberry, pear, grape Citrus Fruit grapefruit, lemon, lime (juice or zest?) Stone Fruit peach, apricot, nectarine Tropical Fruit banana, lychee, mango, melon, passion fruit, pineapple Red Fruit redcurrant, -

Download Authenticated
Ohio Administrative Code Rule 1501:18-1-03 Endangered and threatened species. Effective: January 30, 2021 (A) The following species of plants are designated as endangered in Ohio. (1) Acer pensylvanicum L., Striped maple. (2) Aconitum noveboracense A. Gray, Northern monkshood. (3) Aconitum uncinatum L., Southern monkshood. (4) Adlumia fungosa (Ait.) Greene, Allegheny-vine. (5) Agalinis auriculata (Michx.) Blake, Ear-leaved-foxglove. (6) Agalinis purpurea (L.) Pennell var. parviflora (Benth.) Boivin, Small purple-foxglove. (7) Agalinis skinneriana (Wood) Britt., Skinner's-foxglove. (8) Ageratina aromatica (L.) Spach, Small white snakeroot. (9) Agrostis elliottiana Schultes, Elliott's bent grass. (10) Amelanchier humilis Wiegand, Low serviceberry. (11) Amelanchier interior E.L. Nielsen, Inland serviceberry. (12) Amphidium mougeotii (Bruch, Schimper and W. Gmbel) Schimper, Mougeot's ice moss. (13) Andropogon glomeratus (Walter) Britton, Bushy broom-sedge. Page 1 (14) Androsace occidentalis Pursh, Western rock-jasmine. (15) Anomobryum filiforme (Dicks.) Solms, Common silver moss. (16) Anomodon viticulosus (Hedw.) Hook. and Taylor, Long tail moss. (17) Arabidopsis lyrata (L.) OKane and Al-Shehbaz, Lyre-leaved rock cress. (18) Arabis patens Sullivant, Spreading rock cress. (19) Arctostaphylos uva-ursi (L.) Spreng., Bearberry. (20) Aralia hispida Vent., Bristly sarsaparilla. (21) Arethusa bulbosa L., Dragon's-mouth. (22) Aristida basiramea Engelm. ex Vasey, Forked three-awn grass. (23) Aristida necopina Shinners, False arrow-feather. (24) Aronia arbutifolia (L.) Pers., Red chokeberry. (25) Asplenium bradleyi D.C. Eaton, Bradley's spleenwort. (26) Asplenium resiliens Kunze, Black-stemmed spleenwort. (27) Astragalus neglectus (T. and G.) Sheld., Cooper's milk-vetch. (28) Baptisia australis (L.) R. Br., Blue false indigo. (29) Barbula indica (Hooker) Sprengel in E.G. -

Download This Article in PDF Format
E3S Web of Conferences 254, 02009 (2021) https://doi.org/10.1051/e3sconf/202125402009 FARBA 2021 Use of SSR markers to study genetic polymorphism in members of the Ribes L. genus from the VNIISPK collection Anna Pavlenko*, Maria Dolzhikova, Anna Pikunova, and Annastasiya Bakhotskaya Russian Research Institute of Fruit Crop Breeding (VNIISPK), Zhilina, Orel, Russian Federation Abstract. This study was the first time in Russia to carry out a large-scale (127 samples) assessment of the diverse gene pool of black currant and red currant, the study of intervarietal polymorphism between varieties of the Ribes L. genus from the VNIISPK collection. A cluster analysis of the genetic similarity of black currant varieties (53 varieties), red currant (73 varieties) and one gooseberry variety was performed using 14 SSR markers. Based on the data obtained, a dendrogram was built, in which a number of clusters with high bootstrap support (BS, %) were distinguished. At BS>50%, the coefficients of pairwise genetic similarity between varieties varied from 0.4 to 0.91. The studied representatives of the Ribes genus merged into two main clusters of red currant and black currant. The blackcurrant cluster was joined by gooseberries. The SSR analysis method allows to reveal broad perspectives in the field of identifying varieties affiliation to species. 1 Introduction Currant became known to people quite a long time ago. The first records of black currant were made in the 17th century in the United States by herbalists who drew attention to the medicinal properties of the shrub's fruits and leaves [1]. In wildlife conditions, all Ribes L. -

PASTRY Retail Product List Year 2020
2020 PASTRY Retail Product List Year 2020 www.euraco.com.sg Prices are subject to changes without prior notice Prices are subjected to prevailing GST Please contact Jia Jia Tel : 6276 5433 Fax: 6276 2978 for any orders Email: [email protected] ; [email protected] PASTRY PRODUCTS AVAILABLE EX-STOCK INDEX OF PRODUCTS PAGE VALRHONA 5 - 24 French Chocolate Couvertures Vintage & Aromatised Couvertures / Chocolate Glaze & Other Products Structura Ready-To-Garnish Shells / Truffle Shell Retail Line / Vintage Chocolate / Gastromomie Range / Assorted Pralines HEFTI 25 Pastry Tartlette Shells ALIPRO 26 Fruit Glazing Gels / Oven-Proof Marmalades / Fruit Filling / Snow Powder GELINA 26 Ice Cream & Sherbert Powder SABATON 27 Chestnut & Candied Fruits DGF 28 - 31 Industrial Chocolate Couverture / Decoration & Coating Almond & Baking Paste / Custard Cream / Fruits Glazing Gels Technical Sugar / Corn Syrup / Canned Fruits / MISC DAWN / CAULLET / PELLORCE & JULIEN 32 Fruit & Glazing Gels / Icings Fruit Paste SEVAROME 33 Flavouring Paste / Compound Stabilizer MARIVANI / PROVA 33 Vanilla Bean / Vanilla Extract Concentrated / Pistachio Nuts JUPE / POWDER COLLECTION 34 Concentrated Flavouring Paste / Tea Powder For Bakery & Pastry FABBRI 35 - 40 Gelato Powder / Paste / Marbling / Topping BROVER 41 Fruits In Tin CANE SUGAR AND MISCELLANEOUS 42 - 46 La Perruche Deroche Louis Francois DECO RELIEF 47 - 51 Deco & Sugar Works Décor Powder PAVONI 52 Velvet Dolce Spray CHEF RUBBER 52 Décor Powder VALRHONA 53 Signature Gold Silver Powders and Sprinkles PASTRY -

Vinology Seasonal Desserts
Vinology Seasonal Desserts VEGAN CHOCOLATE & COCONUT “CHEESECAKE” mango passion fruit sorbet, macadamia nut brittle 10 BLOOD ORANGE CREME BRULEE macdamia nut meringue, grapefruit, cocoa nibs, tangerine micro greens 9 PINOT NOIR POACHED PEAR puff pastry, pistachio frangipane, pinot syrup, basil, buttermilk ice cream 11 PB & J chocolate cake, molten peanut butter, malted milk anglaise, concord grape ice cream 10 “KEY LIME” FLOAT buttermilk ice cream, ginger ale 6 PINEAU DES CHARANTES, PORT, HARDY’S CHATEAU D’ORIGNAC ‘WHISKERS BLAKE’ TAWNY subtle hints of ripe apricot, honey, raisins, aged for 8 years this smooth, nutty port produced in Australia white flowers and orange peel 9 is made primarily from Grenache and Shiraz 6 EVOLUCIO, LATE HARVEST TOKAJ PORT, NOVAL RUBY botrytis infected furmint from Hungary, notes of peaches, deep-colored, lively, peppery wine that is well balanced with apricots and white flowers, elegant with nice acidity 7 intense fresh fruit and good length 7 RED RASPBERRY WINE, BERGDORF’S PORT, QUINTA DE LA ROSA produced just outside of Michigan’s capital LATE BOTTLE VINTAGE 2011 features stewed fruit, tart raspberry jam and a clean finish 7 refined with dark plum, dried blackberry, mineral and tar, with hints of dried mint, cedar and chocolate notes 9 SAUTERNES, 2010 LA FLEUR D’OR golden yellow, with the scent of dried apricots and figs, honey PORT, TAYLOR FLADGATE and vanilla, a nicely ballanced full body with a long finish 12 LATE BOTTLED VINTAGE 2010 beautifully perfumed with lots of peppery notes, heaps of black woodland fruit, dark cherry, plumb, redcurrant, hint of black RIESLING, FROST BITTEN licorice 9 made from post-harvest frozen grapes in Yakima Valley, WA ripe pineapple, summer peach and rich floral notes 7 PORT, FONSECA 20 YEAR TAWNY amber color with ripe, plummy, mature fruit, cinnamon, butterscotch, subtle oak and a velvety texture 12. -

Wines by the Glass
Wines By The Glass SOMMELIER RARE SELECTIONS 6oz / 3oz Cabernet Sauvignon, Shafer One Point Five Cabernet Sauvignon, Double Diamond by Shrader Cellars Stags Leap District, Napa Valley, 2015 Oakville, Napa Valley, 2016 An elegant Cabernet with soft tannins and abundant blackberry fruit. Approachable, It has vibrant scent of dark cherry and blackberry, subtly layered with warm notes of vanilla. with dark fruit flavors and silky notes of sweet oak and toasted spices. The palate is explosive, bright and balanced with cassis at the center, with flourishes of cocoa Cabernet Sauvignon, Faust Napa Valley, 2016 and sweet tobacco. Delicate aromas of fresh cranberry and red cherry mingle with enticing notes of Cabernet Sauvignon, Adaptation by Odette, Napa Valley, 2016 cassis, chocolate and tobacco. The wine is round, soft and refined. Intense aromas of ripe plum, currants and black cherry, married with vanilla spice, Cabernet Sauvignon, Artemis, Stag’s Leap Wine Cellars, earth, and coffee. Stags Leap District, Napa Valley, California, 2016 Pinot Noir, Cherry Pie, Stanley Ranch Carneros, Napa Valley, 2014, It opens with intriguing plum, ripe fig and allspice aromas. On the palate, Complex, fruit forward and rich, with layers of ripe strawberry, plum and strawberry, the wine offers flavors of ripe blackberry, chocolate, cherry and hints of cedar. finishing with notes of cola, vanilla and baking spice. Red Blend, The Prisoner, by The Prisoner Wine Company, Alpha Omega Red Blend II, Rutherford, Napa Valley, 2016 Napa Valley, 2017 Dark, ripe blueberries and sweet plum on the nose. Powerful oak and toasty vanilla Intense and alluring aroma of ripe plum reduction layered with hints of cocoa powder and cigar box. -

EU Viruses of Ribes L
SCIENTIFIC OPINION ADOPTED: 26 September 2019 doi: 10.2903/j.efsa.2019.5859 Pest categorisation of non-EU viruses of Ribes L. EFSA Panel on Plant Health (PLH), Claude Bragard, Katharina Dehnen-Schmutz, Paolo Gonthier, Marie-Agnes Jacques, Josep Anton Jaques Miret, Annemarie Fejer Justesen, Alan MacLeod, Christer Sven Magnusson, Panagiotis Milonas, Juan A Navas-Cortes, Stephen Parnell, Roel Potting, Philippe Lucien Reignault, Hans-Hermann Thulke, Wopke Van der Werf, Antonio Vicent Civera, Jonathan Yuen, Lucia Zappala, Thierry Candresse, Elisavet Chatzivassiliou, Franco Finelli, Stephan Winter, Domenico Bosco, Michela Chiumenti, Francesco Di Serio, Franco Ferilli, Tomasz Kaluski, Angelantonio Minafra and Luisa Rubino Abstract Following a request from the EU Commission, the Panel on Plant Health addressed the pest categorisation of the viruses of Ribes L. determined as being either non-EU or of undetermined standing in a previous EFSA opinion. These infectious agents belong to different genera and are heterogeneous in their biology. Alaska vitivirus 1 and Ribes virus F were excluded from categorisation because these are very poorly characterised viruses. The pest categorisation was completed for seven viruses with clear identity and for which detection methods are available. All these viruses are efficiently transmitted by vegetative propagation techniques, with plants for planting representing the major pathway for long- distance dispersal and thus considered as the major pathway for entry. Depending on the virus, additional pathway(s) can also be Ribes seeds, pollen and/or vector(s). Most of the viruses categorised here are known to infect only one or few plant genera, but tomato ringspot virus (ToRSV) has a wide host range, thus extending the possible entry pathways. -

Wines by the Glass
Wines By The Glass SOMMELIER RARE SELECTIONS Cabernet Sauvignon, Nickel & Nickel, “Single Vineyard Selection”, Cabernet Sauvignon, Caymus, Rutherford, Napa Valley, California 2014 Napa Valley, California It has vibrant scent of dark cherry and blackberry, subtly layered with warm notes From the makers of Far Niente, Nickel & Nickel produces 100% Cabernet Sauvignon of vanilla. The palate is explosive, bright and balanced with cassis at the center, wines from select single vineyards throughout Napa Valley. Our Nickel & Nickel with flourishes of cocoa and sweet tobacco. selections benefit from long hang times from warmer sites in Oakville and Rutherford, Red Blend, The Prisoner Wine Company, “The Prisoner”, and are always aged in 100% French Oak. Napa Valley, California, 2014 Cabernet Sauvignon, Jordan, Alexander Valley, Sonoma, California, 2012 The wine has an intense and alluring aroma of ripe plum reduction layered with Delicate aromas of fresh cranberry and red cherry mingle with enticing notes of baking spices, hints of cocoa powder and cigar box. The palette is velvety and cassis, chocolate and tobacco. The wine is round, soft and refined. dense with flavors of black cherry, cola, and freshly roasted coffee beans. Cabernet Sauvignon, Stag’s Leap Wine Cellars, “Artemis”, Cabernet Savignon, Crossbarn by Paul Hobbs, Napa, California 2013 Stags Leap District, Napa Valley, California, 2013 Dense and crimson in color, this superbly balanced wine offers wild aromas of berries, It opens with intriguing plum, ripe fig and allspice aromas. On the palate, clove and black tea on the nose, along with savory herbs and game on the palate, all the wine offers flavors of ripe blackberry, chocolate, cherry and hints of cedar. -

Identification of the 100 Richest Dietary Sources of Polyphenols: an Application of the Phenol-Explorer Database
European Journal of Clinical Nutrition (2010) 64, S112–S120 & 2010 Macmillan Publishers Limited All rights reserved 0954-3007/10 www.nature.com/ejcn ORIGINAL ARTICLE Identification of the 100 richest dietary sources of polyphenols: an application of the Phenol-Explorer database JPe´rez-Jime´nez1,2, V Neveu1,2,FVos1,2 and A Scalbert1,2 1Clermont Universite´, Universite´ d’Auvergne, Unite´ de Nutrition Humaine, Saint-Genes-Champanelle, France and 2INRA, UMR 1019, UNH, CRNH Auvergne, Saint-Genes-Champanelle, France Background/Objectives: The diversity of the chemical structures of dietary polyphenols makes it difficult to estimate their total content in foods, and also to understand the role of polyphenols in health and the prevention of diseases. Global redox colorimetric assays have commonly been used to estimate the total polyphenol content in foods. However, these assays lack specificity. Contents of individual polyphenols have been determined by chromatography. These data, scattered in several hundred publications, have been compiled in the Phenol-Explorer database. The aim of this paper is to identify the 100 richest dietary sources of polyphenols using this database. Subjects/Methods: Advanced queries in the Phenol-Explorer database (www.phenol-explorer.eu) allowed retrieval of information on the content of 502 polyphenol glycosides, esters and aglycones in 452 foods. Total polyphenol content was calculated as the sum of the contents of all individual polyphenols. These content values were compared with the content of antioxidants estimated using the Folin assay method in the same foods. These values were also extracted from the same database. Amounts per serving were calculated using common serving sizes.