Antimicrobial Effect of Natural Berry Juices on Common Oral Pathogenic Bacteria
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Raby Redcurrant Crumble Tray Bake
Raby Redcurrant Crumble tray bake Preparation total time 1 hour Makes 12 portion Nutrition: 100g per serving Kcal Fat Saturates Carbs Sugars Fibre Protein Salt 334 9.41g 5.88g 60.68g 34.92g 1.17g 3.07g 0.40g A layered traybake inspired by German Coffee shops. Ingredients For the berry filling • 200gr Raby red currants • 50g Caster Sugar • 2 tbsp Corn flour For the Cake • 220g Plain Flour • 4g Baking Powder • 2g Cinnamon • 50g Ground Almonds • 200g Caster Sugar • 80g Butter, Melted • 2 Free- range eggs • 40g Yoghurt- thinned with 2tbs milk Method 1. Preheat the oven to 180C/350F/Gas 4. Grease and line a rectangular baking tin (approximately 26cm x 20cm/10½in x 8in). 2. In a saucepan combine the fruit with 50ml/2fl oz water. Bring the fruit just to the boil, reduce the heat and simmer for 2-3 minutes. 3. In a mixing bowl combine the caster sugar with the cornflour. Stir into the fruit and continue to cook for 2-3 minutes, stirring frequently, until the mixture is thick and jammy. Set aside to cool. 4. For the crumble topping, add the flour to a mixing bowl and rub in the butter until the mixture resembles fine breadcrumbs and no large lumps of butter are left. Stir in the sugar and set aside. 5. For the cake, in a large mixing bowl, sift together the flour, baking powder and cinnamon. Stir in the ground almonds until thoroughly combined. 6. In another bowl beat together the sugar with the melted butter, eggs, yoghurt and milk mixture until thoroughly mixed. -

Provision of Information on the Amount of Sugar in Processed Foods
DOI: 10.1590/1413-81232021263.07872019 1153 Provision of information on the amount of sugar FREE THEMES in processed foods Camila Cremonezi Japur (https://orcid.org/0000-0003-0513-1758) 1,4 Dyessa Cardoso Bernardes Assunção (https://orcid.org/0000-0003-4711-7508) 2 Raíssa Aparecida Borges Batista (https://orcid.org/0000-0002-9928-5872) 2 Fernanda Rodrigues de Oliveira Penaforte (https://orcid.org/0000-0001-8483-1562) 3,4 Abstract The objective of this study was to assess the provision of information on the amount of sugar and identify the position of sugar in the list of ingredients of processed foods. A cross-sectional study was conducted to analyze all processed tra- ditional and diet/light/zero food products sold in a hypermarket containing the word sugar or sucrose in the list of ingredients. The food labels were read and the position of sugar on the list of ingredients and presence, or absence, of information on the amount of sugar in the nutrition facts table were 1 Divisão de Nutrição recorded. Information on the amount of sugar was e Metabolismo, Departamento de Ciências also requested from the manufacturers by e-mail da Saúde, Faculdade de or telephone. A total of 2,200 food products were Medicina de Ribeirão Preto, assessed, 2,164 (98.4%) of which were tradition- Universidade de São Paulo (USP). Av. Bandeirantes al foods and 36 (1.6%) diet/light/zero foods. The 3900, Monte Alegre. 14049- amount of sugar was declared in only 14.4% and 900 Ribeirão Preto SP 13.9% of these products, respectively (p=0.84). -

Vaccinium Macrocarpon Ait. Family: Ericaceae
Cultivation Notes No. 54 THE RHODE ISLAND WILD PLANT SOCIETY Winter 2011 Cranberry – Vaccinium macrocarpon Ait. Family: Ericaceae Cranberry: a Native Jewel By Linda Lapin Cranberries are indispensable for the winter holidays. Tradition brings them to our holiday turkey dinners, and who doesn't have a linen table cloth with at least a little red stain on it from a wayward serving of cranberry sauce? This Rhode Island native berry may be a little smaller than the commercially grown cranberry varieties but is just as tasty when used in sauces, jellies, sweet breads, pies and stuffings. Cranberry, Vaccinium macrocarpon, grows wild in Rhode Island. Its native range extends from the East Coast to the Central U. S. and Canada, and from Southern Canada in the north to the Appalachians in the south. It is usually found in acid bogs growing in sphagnum along with other ericaceae such as highbush blueberry and black huckleberry. It grows in company with sheep laurel, leatherleaf, pitcher plants, sundews, and saplings of white cedar and red maple. Look for it in the Great Swamp or Diamond Bog. Cranberry was once called crane berry, because the flower resembles the look of a crane’s head and neck with a long sharp beak. Cranes were also observed wading the bogs gobbling up the berries. Other common names are black cranberry, low cranberry, trailing swamp cranberry, bear berry, and bounce berry. (Cranberries really are the best bouncing fruit I know.) A related species is small cranberry (Vaccinium oxycoccus), which is even smaller and cuter. The plant is a low-growing, evergreen perennial with trailing, wiry stems. -
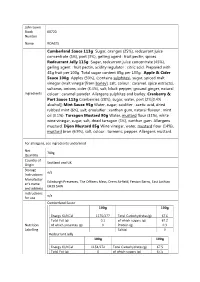
Cumberland Sauce 113G Sugar, Oranges (25%), Redcurrant Juice Concentrate (5%), Port (3%), Gelling Agent : Fruit Pectin, Spices
John Lewis Stock 60720 Number Name ROASTS Cumberland Sauce 113g Sugar, oranges (25%), redcurrant juice concentrate (5%), port (3%), gelling agent : fruit pectin, spices. Redcurrant Jelly 113g Sugar, redcurrant juice concentrate (45%), gelling agent : fruit pectin, acidity regulator : citric acid. Prepared with 45g fruit per 100g. Total sugar content 65g per 100g. Apple & Cider Sauce 100g Apples (50%), (contains sulphites), sugar, spiced malt vinegar (malt vinegar[from barley], salt, colour : caramel, spice extracts), sultanas, onions, cider (3.4%), salt, black pepper, ground ginger, natural Ingredients colour : caramel powder. Allergens sulphites and barley. Cranberry & Port Sauce 113g Cranberries (38%), sugar, water, port (2%[0.4% alcohol]) Mint Sauce 95g Water, sugar, acidifier : acetic acid, dried rubbed mint (6%), salt, emulsifier : xanthan gum, natural flavour : mint oil (0.1%). Tarragon Mustard 90g Water, mustard flour (31%), white wine vinegar, sugar, salt, dried tarragon (3%), xanthan gum. Allergens mustard. Dijon Mustard 85g Wine vinegar, water, mustard flour (14%), mustard bran (6.9%), salt, colour : turmeric, pepper. Allergens mustard. For allergens, see ingredients underlined Net 709g Quantity Country of Scotland and UK Origin Storage n/a Instructions Manufactur Edinburgh Preserves, The Officers Mess, Drem Airfield, Fenton Barns, East Lothian er’s name EH39 5AW and address Instructions n/a for use Cumberland Sauce 100g 100g Energy KJ/KCal 1175/277 Total Carbohydrates(g) 67.6 Total Fat (g) 0.1 of which sugars (g) 67.2 Nutrition -

WSET Level 2 Systematic Approach to Tasting Wine®
Level 2 SAT (A5) 2012 (v2)_Layout 1 30/03/2012 15:47 Page 1 WSET Level 2 Systematic Approach to Tasting Wine ® APPEARANCE Clarity clear – hazy Intensity pale – medium – deep Colour white lemon – gold – amber rosé pink – salmon – orange red purple – ruby – garnet – tawny NOSE Condition clean – unclean Intensity light – medium – pronounced Aroma characteristics e.g. fruits, flowers, spices, vegetables, oak aromas, other PALATE Sweetness dry – off-dry – medium – sweet Acidity low – medium – high Tannin low – medium – high Body light – medium – full Flavour characteristics e.g. fruits, flowers, spices, vegetables, oak flavours, other Finish short – medium – long CONCLUSIONS Quality faulty – poor – acceptable – good – very good – outstanding Copyright Wine & Spirit Education Trust 2012. The WSET Level 2 Systematic Approach to Tasting Wine ® may only be reproduced with the written permission of the WSET subject to their terms and conditions. For more information contact [email protected] 2W-Version 1.0 Level 2 SAT (A5) 2012 (v2)_Layout 1 30/03/2012 15:47 Page 2 WSET Level 2 Wine-Lexicon: supporting the WSET Level 2 Systematic Approach to Tasting Wine ® AROMA AND FLAVOUR CHARACTERISTICS FLORAL / FRUIT Are the flavours simple/generic or specific? Fresh or cooked? Ripe or unripe? Floral blossom, rose, violet Green Fruit green apple, red apple, gooseberry, pear, grape Citrus Fruit grapefruit, lemon, lime (juice or zest?) Stone Fruit peach, apricot, nectarine Tropical Fruit banana, lychee, mango, melon, passion fruit, pineapple Red Fruit redcurrant, -

Vaccinium Oxycoccos L
Plant Guide these fruits in their food economy (Waterman 1920). SMALL CRANBERRY Small cranberries were gathered wild in England and Vaccinium oxycoccos L. Scotland and made into tarts, marmalade, jelly, jam, Plant Symbol = VAOX and added to puddings and pies (Eastwood 1856). Many colonists were already familiar with this fruit Contributed by: USDA NRCS National Plant Data in Great Britain before finding it in North America. Team, Greensboro, NC The small cranberry helped stock the larder of English and American ships, fed trappers in remote regions, and pleased the palates of Meriwether Lewis and William Clark in their explorations across the United States (Lewis and Clark 1965). The Chinook, for example, traded dried cranberries with the English vessel Ruby in 1795 and at Thanksgiving in 1805 Lewis and Clark dined on venison, ducks, geese, and small cranberry sauce from fruit brought by Chinook women (McDonald 1966; Lewis and Clark 1965). Because the small cranberry can grow in association with large cranberry (Vaccinium macrocarpon) in the Great Lakes region, northeastern USA and southeastern Canada (Boniello 1993; Roger Latham pers. comm. 2009) it is possible that the Pilgrims of Plymouth were introduced to both edible Small cranberries growing in a bog on the western Olympic species by the Wampanoag. Peninsula, Washington. Photograph by Jacilee Wray, 2006. The berries are still gathered today in the United Alternate Names States, Canada, and Europe (Himelrick 2005). The Bog cranberry, swamp cranberry, wild cranberry Makah, Quinault, and Quileute of the Olympic Peninsula still gather them every fall and non-Indians Uses from early settler families still gather them (Anderson Said to have a superior flavor to the cultivated 2009). -

Download Authenticated
Ohio Administrative Code Rule 1501:18-1-03 Endangered and threatened species. Effective: January 30, 2021 (A) The following species of plants are designated as endangered in Ohio. (1) Acer pensylvanicum L., Striped maple. (2) Aconitum noveboracense A. Gray, Northern monkshood. (3) Aconitum uncinatum L., Southern monkshood. (4) Adlumia fungosa (Ait.) Greene, Allegheny-vine. (5) Agalinis auriculata (Michx.) Blake, Ear-leaved-foxglove. (6) Agalinis purpurea (L.) Pennell var. parviflora (Benth.) Boivin, Small purple-foxglove. (7) Agalinis skinneriana (Wood) Britt., Skinner's-foxglove. (8) Ageratina aromatica (L.) Spach, Small white snakeroot. (9) Agrostis elliottiana Schultes, Elliott's bent grass. (10) Amelanchier humilis Wiegand, Low serviceberry. (11) Amelanchier interior E.L. Nielsen, Inland serviceberry. (12) Amphidium mougeotii (Bruch, Schimper and W. Gmbel) Schimper, Mougeot's ice moss. (13) Andropogon glomeratus (Walter) Britton, Bushy broom-sedge. Page 1 (14) Androsace occidentalis Pursh, Western rock-jasmine. (15) Anomobryum filiforme (Dicks.) Solms, Common silver moss. (16) Anomodon viticulosus (Hedw.) Hook. and Taylor, Long tail moss. (17) Arabidopsis lyrata (L.) OKane and Al-Shehbaz, Lyre-leaved rock cress. (18) Arabis patens Sullivant, Spreading rock cress. (19) Arctostaphylos uva-ursi (L.) Spreng., Bearberry. (20) Aralia hispida Vent., Bristly sarsaparilla. (21) Arethusa bulbosa L., Dragon's-mouth. (22) Aristida basiramea Engelm. ex Vasey, Forked three-awn grass. (23) Aristida necopina Shinners, False arrow-feather. (24) Aronia arbutifolia (L.) Pers., Red chokeberry. (25) Asplenium bradleyi D.C. Eaton, Bradley's spleenwort. (26) Asplenium resiliens Kunze, Black-stemmed spleenwort. (27) Astragalus neglectus (T. and G.) Sheld., Cooper's milk-vetch. (28) Baptisia australis (L.) R. Br., Blue false indigo. (29) Barbula indica (Hooker) Sprengel in E.G. -

Download This Article in PDF Format
E3S Web of Conferences 254, 02009 (2021) https://doi.org/10.1051/e3sconf/202125402009 FARBA 2021 Use of SSR markers to study genetic polymorphism in members of the Ribes L. genus from the VNIISPK collection Anna Pavlenko*, Maria Dolzhikova, Anna Pikunova, and Annastasiya Bakhotskaya Russian Research Institute of Fruit Crop Breeding (VNIISPK), Zhilina, Orel, Russian Federation Abstract. This study was the first time in Russia to carry out a large-scale (127 samples) assessment of the diverse gene pool of black currant and red currant, the study of intervarietal polymorphism between varieties of the Ribes L. genus from the VNIISPK collection. A cluster analysis of the genetic similarity of black currant varieties (53 varieties), red currant (73 varieties) and one gooseberry variety was performed using 14 SSR markers. Based on the data obtained, a dendrogram was built, in which a number of clusters with high bootstrap support (BS, %) were distinguished. At BS>50%, the coefficients of pairwise genetic similarity between varieties varied from 0.4 to 0.91. The studied representatives of the Ribes genus merged into two main clusters of red currant and black currant. The blackcurrant cluster was joined by gooseberries. The SSR analysis method allows to reveal broad perspectives in the field of identifying varieties affiliation to species. 1 Introduction Currant became known to people quite a long time ago. The first records of black currant were made in the 17th century in the United States by herbalists who drew attention to the medicinal properties of the shrub's fruits and leaves [1]. In wildlife conditions, all Ribes L. -

PASTRY Retail Product List Year 2020
2020 PASTRY Retail Product List Year 2020 www.euraco.com.sg Prices are subject to changes without prior notice Prices are subjected to prevailing GST Please contact Jia Jia Tel : 6276 5433 Fax: 6276 2978 for any orders Email: [email protected] ; [email protected] PASTRY PRODUCTS AVAILABLE EX-STOCK INDEX OF PRODUCTS PAGE VALRHONA 5 - 24 French Chocolate Couvertures Vintage & Aromatised Couvertures / Chocolate Glaze & Other Products Structura Ready-To-Garnish Shells / Truffle Shell Retail Line / Vintage Chocolate / Gastromomie Range / Assorted Pralines HEFTI 25 Pastry Tartlette Shells ALIPRO 26 Fruit Glazing Gels / Oven-Proof Marmalades / Fruit Filling / Snow Powder GELINA 26 Ice Cream & Sherbert Powder SABATON 27 Chestnut & Candied Fruits DGF 28 - 31 Industrial Chocolate Couverture / Decoration & Coating Almond & Baking Paste / Custard Cream / Fruits Glazing Gels Technical Sugar / Corn Syrup / Canned Fruits / MISC DAWN / CAULLET / PELLORCE & JULIEN 32 Fruit & Glazing Gels / Icings Fruit Paste SEVAROME 33 Flavouring Paste / Compound Stabilizer MARIVANI / PROVA 33 Vanilla Bean / Vanilla Extract Concentrated / Pistachio Nuts JUPE / POWDER COLLECTION 34 Concentrated Flavouring Paste / Tea Powder For Bakery & Pastry FABBRI 35 - 40 Gelato Powder / Paste / Marbling / Topping BROVER 41 Fruits In Tin CANE SUGAR AND MISCELLANEOUS 42 - 46 La Perruche Deroche Louis Francois DECO RELIEF 47 - 51 Deco & Sugar Works Décor Powder PAVONI 52 Velvet Dolce Spray CHEF RUBBER 52 Décor Powder VALRHONA 53 Signature Gold Silver Powders and Sprinkles PASTRY -

Vinology Seasonal Desserts
Vinology Seasonal Desserts VEGAN CHOCOLATE & COCONUT “CHEESECAKE” mango passion fruit sorbet, macadamia nut brittle 10 BLOOD ORANGE CREME BRULEE macdamia nut meringue, grapefruit, cocoa nibs, tangerine micro greens 9 PINOT NOIR POACHED PEAR puff pastry, pistachio frangipane, pinot syrup, basil, buttermilk ice cream 11 PB & J chocolate cake, molten peanut butter, malted milk anglaise, concord grape ice cream 10 “KEY LIME” FLOAT buttermilk ice cream, ginger ale 6 PINEAU DES CHARANTES, PORT, HARDY’S CHATEAU D’ORIGNAC ‘WHISKERS BLAKE’ TAWNY subtle hints of ripe apricot, honey, raisins, aged for 8 years this smooth, nutty port produced in Australia white flowers and orange peel 9 is made primarily from Grenache and Shiraz 6 EVOLUCIO, LATE HARVEST TOKAJ PORT, NOVAL RUBY botrytis infected furmint from Hungary, notes of peaches, deep-colored, lively, peppery wine that is well balanced with apricots and white flowers, elegant with nice acidity 7 intense fresh fruit and good length 7 RED RASPBERRY WINE, BERGDORF’S PORT, QUINTA DE LA ROSA produced just outside of Michigan’s capital LATE BOTTLE VINTAGE 2011 features stewed fruit, tart raspberry jam and a clean finish 7 refined with dark plum, dried blackberry, mineral and tar, with hints of dried mint, cedar and chocolate notes 9 SAUTERNES, 2010 LA FLEUR D’OR golden yellow, with the scent of dried apricots and figs, honey PORT, TAYLOR FLADGATE and vanilla, a nicely ballanced full body with a long finish 12 LATE BOTTLED VINTAGE 2010 beautifully perfumed with lots of peppery notes, heaps of black woodland fruit, dark cherry, plumb, redcurrant, hint of black RIESLING, FROST BITTEN licorice 9 made from post-harvest frozen grapes in Yakima Valley, WA ripe pineapple, summer peach and rich floral notes 7 PORT, FONSECA 20 YEAR TAWNY amber color with ripe, plummy, mature fruit, cinnamon, butterscotch, subtle oak and a velvety texture 12. -

Wines by the Glass
Wines By The Glass SOMMELIER RARE SELECTIONS 6oz / 3oz Cabernet Sauvignon, Shafer One Point Five Cabernet Sauvignon, Double Diamond by Shrader Cellars Stags Leap District, Napa Valley, 2015 Oakville, Napa Valley, 2016 An elegant Cabernet with soft tannins and abundant blackberry fruit. Approachable, It has vibrant scent of dark cherry and blackberry, subtly layered with warm notes of vanilla. with dark fruit flavors and silky notes of sweet oak and toasted spices. The palate is explosive, bright and balanced with cassis at the center, with flourishes of cocoa Cabernet Sauvignon, Faust Napa Valley, 2016 and sweet tobacco. Delicate aromas of fresh cranberry and red cherry mingle with enticing notes of Cabernet Sauvignon, Adaptation by Odette, Napa Valley, 2016 cassis, chocolate and tobacco. The wine is round, soft and refined. Intense aromas of ripe plum, currants and black cherry, married with vanilla spice, Cabernet Sauvignon, Artemis, Stag’s Leap Wine Cellars, earth, and coffee. Stags Leap District, Napa Valley, California, 2016 Pinot Noir, Cherry Pie, Stanley Ranch Carneros, Napa Valley, 2014, It opens with intriguing plum, ripe fig and allspice aromas. On the palate, Complex, fruit forward and rich, with layers of ripe strawberry, plum and strawberry, the wine offers flavors of ripe blackberry, chocolate, cherry and hints of cedar. finishing with notes of cola, vanilla and baking spice. Red Blend, The Prisoner, by The Prisoner Wine Company, Alpha Omega Red Blend II, Rutherford, Napa Valley, 2016 Napa Valley, 2017 Dark, ripe blueberries and sweet plum on the nose. Powerful oak and toasty vanilla Intense and alluring aroma of ripe plum reduction layered with hints of cocoa powder and cigar box. -

Sparkling™ Juice Drinks
Sparkling™ Juice Drinks Made with 15% real No artificial juice 1/2 amount of fruit juice 70 sweeteners or sugar vs. calories preservatives SRP: per 11.5 oz. Good leading soft serving source of $1.79 drink* Vitamin C 4 fun bubbly flavors for on-the-go! *This product contains 17g sugar per 11.5 FL OZ serving compared to 37g sugar in 11.5 FL OZ serving of the leading soft drink Ocean Spray® Sparkling™ Juice Drinks Made with 15% real No artificial juice 1/2 amount of fruit juice 70 sweeteners or sugar vs. calories preservatives SRP: per 11.5 oz. Good leading soft serving source of $1.79 drink* Vitamin C CASE INFORMATION | 104/6/4/11.5oz Gross Net Volume Length Width Height Spec Pack Weight Weight (CI/CF) (in) (in) (in) (LBS) (LBS) Can (11.5oz) 1 0.811 0.763 31.294 CI 2.260 2.260 6.127 4-Pack 124.659 CI 4 3.350 3.054 4.500 4.500 6.156 (4/11.5oz) Case 6 20.300 18.324 0.489 CF 14.125 9.375 6.375 (6/4/11.5oz) Pallet 104 2,119.86 1,905.68 52.192 CF 47.000 37.625 51.000 (104/6/4/11.5oz) NUTRITIONAL INFORMATION Total Total Case Sugars Protein Vitamin C Sodium Description Cal. Fat Carbs( UPC (g) (g) (mg) (mg) (g) g) 21399 Sparkling™ Cranberry Raspberry 70 0 20 17 0 18 10 21398 Sparkling™ Cranberry Mango 70 0 19 17 0 18 10 22717 Sparkling™ Cranberry 70 0 20 17 0 18 10 21356 Sparkling™ Diet Cranberry 10 0 4 1 0 18 65 Ingredients: Sparkling™ Cranberry Raspberry: Sparkling Water, Grape Juice (water, grape juice concentrate), Cranberry Juice (water, cranberry juice concentrate), Sugar, Raspberry Juice (water, raspberry juice concentrate), Natural Flavor,