Volltext (PDF)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lobsters-Identification, World Distribution, and U.S. Trade
Lobsters-Identification, World Distribution, and U.S. Trade AUSTIN B. WILLIAMS Introduction tons to pounds to conform with US. tinents and islands, shoal platforms, and fishery statistics). This total includes certain seamounts (Fig. 1 and 2). More Lobsters are valued throughout the clawed lobsters, spiny and flat lobsters, over, the world distribution of these world as prime seafood items wherever and squat lobsters or langostinos (Tables animals can also be divided rougWy into they are caught, sold, or consumed. 1 and 2). temperate, subtropical, and tropical Basically, three kinds are marketed for Fisheries for these animals are de temperature zones. From such partition food, the clawed lobsters (superfamily cidedly concentrated in certain areas of ing, the following facts regarding lob Nephropoidea), the squat lobsters the world because of species distribu ster fisheries emerge. (family Galatheidae), and the spiny or tion, and this can be recognized by Clawed lobster fisheries (superfamily nonclawed lobsters (superfamily noting regional and species catches. The Nephropoidea) are concentrated in the Palinuroidea) . Food and Agriculture Organization of temperate North Atlantic region, al The US. market in clawed lobsters is the United Nations (FAO) has divided though there is minor fishing for them dominated by whole living American the world into 27 major fishing areas for in cooler waters at the edge of the con lobsters, Homarus americanus, caught the purpose of reporting fishery statis tinental platform in the Gul f of Mexico, off the northeastern United States and tics. Nineteen of these are marine fish Caribbean Sea (Roe, 1966), western southeastern Canada, but certain ing areas, but lobster distribution is South Atlantic along the coast of Brazil, smaller species of clawed lobsters from restricted to only 14 of them, i.e. -

Factors Affecting Growth of the Spiny Lobsters Panulirus Gracilis and Panulirus Inflatus (Decapoda: Palinuridae) in Guerrero, México
Rev. Biol. Trop. 51(1): 165-174, 2003 www.ucr.ac.cr www.ots.ac.cr www.ots.duke.edu Factors affecting growth of the spiny lobsters Panulirus gracilis and Panulirus inflatus (Decapoda: Palinuridae) in Guerrero, México Patricia Briones-Fourzán and Enrique Lozano-Álvarez Universidad Nacional Autónoma de México, Instituto de Ciencias del Mar y Limnología, Unidad Académica Puerto Morelos. P. O. Box 1152, Cancún, Q. R. 77500 México. Fax: +52 (998) 871-0138; [email protected] Received 00-XX-2002. Corrected 00-XX-2002. Accepted 00-XX-2002. Abstract: The effects of sex, injuries, season and site on the growth of the spiny lobsters Panulirus gracilis, and P. inflatus, were studied through mark-recapture techniques in two sites with different ecological characteristics on the coast of Guerrero, México. Panulirus gracilis occurred in both sites, whereas P. inflatus occurred only in one site. All recaptured individuals were adults. Both species had similar intermolt periods, but P. gracilis had significantly higher growth rates (mm carapace length week-1) than P. inflatus as a result of a larger molt incre- ment. Growth rates of males were higher than those of females in both species owing to larger molt increments and shorter intermolt periods in males. Injuries had no effect on growth rates in either species. Individuals of P. gracilis grew faster in site 1 than in site 2. Therefore, the effect of season on growth of P. gracilis was analyzed separately in each site. In site 2, growth rates of P. gracilis were similar in summer and in winter, whereas in site 1 both species had higher growth rates in winter than in summer. -
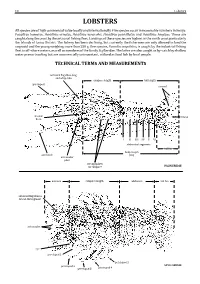
Lobsters LOBSTERS§
18 Lobsters LOBSTERS§ All species are of high commercial value locally and internationally. Five species occur in reasonable numbers in Kenya: Panulirus homarus, Panulirus ornatus, Panulirus versicolor, Panulirus penicillatus and Panulirus longipes. These are caughtungravid along and the the coast young by weighing the artisanal more fishing than 250 fleet. g. Landings One species, of these Puerulus species angulatus are highest in the north coast particularly the Islands of Lamu District. The fishery has been declining,Scyllaridae. but currently The latter the fishermen are also caught are only as by–catch allowed toby landshallow the , is caught by the industrial fishing fleet in off–shore waters, as well as members of the family water prawn trawling but areTECHNICAL commercially unimportant, TERMS AND utilized MEASUREMENTS as food fish by local people. and whip–like antennal flagellum long carapace length tail length pereiopod uropod frontal telson horn III III IV VIV abdominal segments tail fan body length antennule (BL) antennular plate strong spines on carapace PALINURIDAE antenna carapace length abdomen tail fan antennal flagellum a broad, flat segment antennules eye pereiopod 1 pereiopod 5 pereiopod 2 SCYLLARIDAE pereiopod 3 pereiopod 4 Guide to Families 19 GUIDE TO FAMILIES NEPHROPIDAE Page 20 True lobsters § To about 15 cm. Marine, mainly deep waters on soft included in the Guide to Species. 1st pair of substrates. Three species of interest to fisheriespereiopods are large 3rd pair of pereiopods with chela PALINURIDAE Page 21 Antennal Spiny lobsters § To about 50 cm. Marine, mostly shallow waters on flagellum coral and sand stone reefs, some species on soft included in the Guide to Species. -

Balanus Trigonus
Nauplius ORIGINAL ARTICLE THE JOURNAL OF THE Settlement of the barnacle Balanus trigonus BRAZILIAN CRUSTACEAN SOCIETY Darwin, 1854, on Panulirus gracilis Streets, 1871, in western Mexico e-ISSN 2358-2936 www.scielo.br/nau 1 orcid.org/0000-0001-9187-6080 www.crustacea.org.br Michel E. Hendrickx Evlin Ramírez-Félix2 orcid.org/0000-0002-5136-5283 1 Unidad académica Mazatlán, Instituto de Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México. A.P. 811, Mazatlán, Sinaloa, 82000, Mexico 2 Oficina de INAPESCA Mazatlán, Instituto Nacional de Pesca y Acuacultura. Sábalo- Cerritos s/n., Col. Estero El Yugo, Mazatlán, 82112, Sinaloa, Mexico. ZOOBANK http://zoobank.org/urn:lsid:zoobank.org:pub:74B93F4F-0E5E-4D69- A7F5-5F423DA3762E ABSTRACT A large number of specimens (2765) of the acorn barnacle Balanus trigonus Darwin, 1854, were observed on the spiny lobster Panulirus gracilis Streets, 1871, in western Mexico, including recently settled cypris (1019 individuals or 37%) and encrusted specimens (1746) of different sizes: <1.99 mm, 88%; 1.99 to 2.82 mm, 8%; >2.82 mm, 4%). Cypris settled predominantly on the carapace (67%), mostly on the gastric area (40%), on the left or right orbital areas (35%), on the head appendages, and on the pereiopods 1–3. Encrusting individuals were mostly small (84%); medium-sized specimens accounted for 11% and large for 5%. On the cephalothorax, most were observed in branchial (661) and orbital areas (240). Only 40–41 individuals were found on gastric and cardiac areas. Some individuals (246), mostly small (95%), were observed on the dorsal portion of somites. -

Redalyc.Occurrence of Panulirus Inflatus (Decapoda: Palinuridae
Revista de Biología Marina y Oceanografía ISSN: 0717-3326 [email protected] Universidad de Valparaíso Chile Pérez-González, Raúl; Puga, Dagoberto; Valadez, Luis M.; Rodríguez-Domínguez, Guillermo Occurrence of Panulirus inflatus (Decapoda: Palinuridae) pueruli in the southeastern Gulf of California, Mexico Revista de Biología Marina y Oceanografía, vol. 51, núm. 1, abril, 2016, pp. 223-227 Universidad de Valparaíso Viña del Mar, Chile Available in: http://www.redalyc.org/articulo.oa?id=47945599023 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Revista de Biología Marina y Oceanografía Vol. 51, Nº1: 209-215, abril 2016 DOI 10.4067/S0718-19572016000100023 RESEARCH NOTE Occurrence of Panulirus inflatus (Decapoda: Palinuridae) pueruli in the southeastern Gulf of California, Mexico Presencia de puérulos de Panulirus inflatus (Bouvier, 1895) (Decapoda: Palinuridae) en el sureste del golfo de California, México Raúl Pérez-González1, Dagoberto Puga2, Luis M. Valadez1 and Guillermo Rodríguez-Domínguez1 1Facultad de Ciencias del Mar, Universidad Autónoma de Sinaloa, Paseo Claussen s/n, C.P. 82000, Apdo. Postal 610, Mazatlán, Sinaloa, México. [email protected] 2Instituto Nacional de Pesca, Centro Regional de Investigación Pesquera de Bahía Banderas, Nayarit. Calle Tortuga No. 1, La Cruz de Huanacaxtle, C.P. 63732, Nayarit, México Abstract.- This study presents results on the collection of Panulirus inflatus pueruli in seaweed (GuSi; set at the surface) and crevice (Booth: set on the bottom) collectors from April to December 1998 in waters of the southeastern Gulf of California, Mexico. -

Redalyc.Catch Composition of the Spiny Lobster Panulirus Gracilis (Decapoda: Palinuridae) Off the Western Coast of Mexico
Latin American Journal of Aquatic Research E-ISSN: 0718-560X [email protected] Pontificia Universidad Católica de Valparaíso Chile Pérez-González, Raúl Catch composition of the spiny lobster Panulirus gracilis (Decapoda: Palinuridae) off the western coast of Mexico Latin American Journal of Aquatic Research, vol. 39, núm. 2, julio, 2011, pp. 225-235 Pontificia Universidad Católica de Valparaíso Valparaiso, Chile Available in: http://www.redalyc.org/articulo.oa?id=175019398004 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Lat. Am. J. Aquat. Res., 39(2): 225-235, 2011 Population structure of Panulirus gracilis 225 DOI: 10.3856/vol39-issue2-fulltext-4 Research Article Catch composition of the spiny lobster Panulirus gracilis (Decapoda: Palinuridae) off the western coast of Mexico Raúl Pérez-González Universidad Autónoma de Sinaloa, Facultad de Ciencias del Mar P.O. Box 610, Mazatlán, Sinaloa, México ABSTRACT. The lobster fishery in the Gulf of California and the south-central region of the western coast of Mexico consists of small-scale artisanal activity supported by Panulirus gracilis and P. inflatus, with an annual average catch of 132 ton. The present study analyzes the landing composition of this fishery and the population structure of P. gracilis. Carapace lengths (CL) for this species ranged from 35 to 125 mm, and the most frequent sizes were between 60 and 85 mm. The size distribution was approximately normal. This implies that the fishery is composed of several size classes, with annual recruitment to the fishing areas. -

ESTADÍSTICO DE PESCA Índice
ANUARIO ESTADÍSTICO DE PESCA Índice INTRODUCCIÓN 7 CAPÍTULO I PRODUCCIÓN PESQUERA 13 CAPÍTULO II INDUSTRIALIZACIÓN 109 CAPÍTULO III COMERCIALIZACIÓN Y CONSUMO 123 CAPÍTULO IV FACTORES DE PRODUCCIÓN 147 CAPÍTULO V NORMATIVIDAD 177 CAPÍTULO VI ESTADÍSTICAS INTERNACIONALES 201 GLOSARIO 237 ÍNDICE DE CUADROS 243 ANEXO 257 SECRETARÍA DE AGRICULTURA, GANADERÍA, DESARROLLO RURAL, PESCA Y ALIMENTACIÓN Javier Bernardo Usabiaga Arroyo SECRETARIO Jerónimo Ramos Sáenz Pardo COMISIONADO NACIONAL DE ACUACULTURA Y PESCA Juan Carlos Cortés García SUBSECRETARIO DE PLANEACIÓN Víctor Villalobos Arámbula SUBSECRETARIO DE AGRICULTURA Y GANADERÍA Antonio Ruiz García SUBSECRETARIO DE DESARROLLO RURAL Mara Angélica Murillo Correa DIRECTORA GENERAL DE POLÍTICA Y FOMENTO PESQUERO Guillermo Compean Jiménez PRESIDENTE DEL INSTITUTO NACIONAL DE LA PESCA p Introducción Introducción a Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación, tiene como uno de sus propósitos esenciales difundir en forma confiable y oportuna, los L principales indicadores de la actividad pesquera en México, que son importantes para conocer el comportamiento y evolución de la explotación, conservación e industrialización de la flora y fauna acuática del país. La SAGARPA a través del desarrollo y actualización de su infraestructura informática y el rediseño de los sistemas estadísticos, aunado a la automatización en sus procesos, propicia las condiciones necesarias para la generación de información estadística actual y confiable, que permite conocer los fenómenos que comprende la pesca en su conjunto. Para la integración de este documento fue necesaria una cercana vinculación entre las delegaciones federales, las oficinas de la SAGARPA y los órganos centrales de la Secretaría, quienes por medio de procedimiento ya establecido, llevaron a cabo la tarea de recopilar e integrar la información estadística emanada de los diferentes agentes que participan activamente en este sector. -

Goldstein Et Al 2019
Journal of Crustacean Biology Advance Access published 24 August 2019 Journal of Crustacean Biology The Crustacean Society Journal of Crustacean Biology 39(5), 574–581, 2019. doi:10.1093/jcbiol/ruz055 Downloaded from https://academic.oup.com/jcb/article-abstract/39/5/574/5554142/ by University of New England Libraries user on 04 October 2019 Development in culture of larval spotted spiny lobster Panulirus guttatus (Latreille, 1804) (Decapoda: Achelata: Palinuridae) Jason S. Goldstein1, Hirokazu Matsuda2, , Thomas R. Matthews3, Fumihiko Abe4, and Takashi Yamakawa4, 1Wells National Estuarine Research Reserve, Maine Coastal Ecology Center, 342 Laudholm Farm Road, Wells, ME 04090 USA; 2Mie Prefecture Fisheries Research Institute, 3564-3, Hamajima, Shima, Mie 517-0404 Japan; 3Florida Fish and Wildlife Conservation Commission, Fish and Wildlife Research Institute, 2796 Overseas Hwy, Suite 119, Marathon, FL 33050 USA; and 4Department of Aquatic Bioscience, Graduate School of Agricultual and Life Sciences, The University of Tokyo, 1-1-1 Yayoi, Bunkyo, Tokyo 113-8657 Japan HeadA=HeadB=HeadA=HeadB/HeadA Correspondence: J.S. Goldstein: e-mail: [email protected] HeadB=HeadC=HeadB=HeadC/HeadB (Received 15 May 2019; accepted 11 July 2019) HeadC=HeadD=HeadC=HeadD/HeadC Ack_Text=DisHead=Ack_Text=HeadA ABSTRACT NList_lc_rparentheses_roman2=Extract1=NList_lc_rparentheses_roman2=Extract1_0 There is little information on the early life history of the spotted spiny lobster Panulirus guttatus (Latreille, 1804), an obligate reef resident, despite its growing importance as a fishery re- BOR_HeadA=BOR_HeadB=BOR_HeadA=BOR_HeadB/HeadA source in the Caribbean and as a significant predator. We cultured newly-hatched P. guttatus BOR_HeadB=BOR_HeadC=BOR_HeadB=BOR_HeadC/HeadB larvae (phyllosomata) in the laboratory for the first time, and the growth, survival, and mor- BOR_HeadC=BOR_HeadD=BOR_HeadC=BOR_HeadD/HeadC phological descriptions are reported through 324 days after hatch (DAH). -

Growth Patterns and Exploitation Status of the Spiny Lobster Species Palinurus Mauritanicus (Gruvel 1911) in Mauritanian Coasts
International Journal of Agricultural Policy and Research Vol.7 (2), pp. 17-31, March 2019 Available online at https://www.journalissues.org/IJAPR/ https://doi.org/10.15739/IJAPR.19.003 Copyright © 2019 Author(s) retain the copyright of this article ISSN 2350-1561 Original Research Article Growth patterns and exploitation status of the spiny lobster species Palinurus mauritanicus (Gruvel 1911) in Mauritanian coasts Received 3 January, 2019 Revised 10 February, 2019 Accepted 15 February, 2019 Published 14 March, 2019 Amadou Sow1, In the Mauritanian coasts, fishing effort on Palinurus mauritanicus Bilassé Zongo*2 contributes in the decline of the stock. From February to August 2015, samplings were carried out monthly in order to study the growth patterns and and the stock status to provide further information for sustainable 2 T. Jean André Kabre management and exploitation of the species. A total of 12008 individuals were collected. Total length (Lt) and cephalothoracic length (Lc) were 1Institut Mauritanien des measured with a vernier caliper. The species were separately weighed on a Recherches Océanographiques et digital balance and their sex noted. The collected data were entered on excel des Pêches (IMROP), Mauritanie spreadsheet in order to analyse growth kinetics, exploitation kinetics and 2Université Nazi Boni, LaRFPF, estimate fish mortality using FISAT II software. Male of P. mauritanicus had Burkina Faso an average Lc = 130 mm and an average Lc = 111 mm. The annual length frequency distribution gave respectively 6 and 7 age-groups for females and *Corresponding Author males. Lc-Lt relationship and Lc-Wt relationship showed a minor allometry Email: [email protected] (b < 3 for both). -

Taxonomy, Biology and Distribution of Lobsters
Taxonomy, Biology and Distribution of Lobsters 15 Rekha Devi Chakraborty and E.V.Radhakrishnan Crustacean Fisheries Division, Central Marine Fisheries Research Institute, Kochi-682 018 Lobsters are among the most prized of fisheries resources and of significant commercial interest in many countries. Because of their high value and esteemed culinary worth, much attention has been paid to lobsters in biological, fisheries, and systematic literature. They have a great demand in the domestic market as a delicacy and is a foreign exchange earner for the country. Taxonomic status Phylum: Arthropoda Subphylum: Crustacea Class: Malacostraca Subclass: Eumalacostraca Superorder: Eucarida Order: Decapoda Suborder: Macrura Reptantia The suborder Macrura Reptantia consists of three infraorders: Astacidea (Marine lobsters and freshwater crayfishes), Palinuridea (Spiny lobsters and slipper lobsters) and Thalassinidea (mud lobsters). The infraorder Astacidea Summer School on Recent Advances in Marine Biodiversity Conservation and Management 100 Rekha Devi Chakraborty and E.V.Radhakrishnan contains three superfamilies of which only one (the Infraorder Palinuridea, Superfamily Eryonoidea, Family Nephropoidea) is considered here. The remaining two Polychelidae superfamilies (Astacoidea and parastacoidea) contain the 1b. Third pereiopod never with a true chela,in most groups freshwater crayfishes. The superfamily Nephropoidea (40 chelae also absent from first and second pereiopods species) consists almost entirely of commercial or potentially 3a Antennal flagellum reduced to a single broad and flat commercial species. segment, similar to the other antennal segments ..... Infraorder Palinuridea, Superfamily Palinuroidea, The infraorder Palinuridea also contains three superfamilies Family Scyllaridae (Eryonoidea, Glypheoidea and Palinuroidea) all of which are 3b Antennal flagellum long, multi-articulate, flexible, whip- marine. The Eryonoidea are deepwater species of insignificant like, or more rigid commercial interest. -

Seafood Watch
Spiny Lobster Panulirus interruptus ©B. Guild Gillespie/www.chartingnature.com California Traps December 27, 2012 Meghan Sullivan, Consulting Researcher Disclaimer Seafood Watch® strives to ensure all our Seafood Reports and the recommendations contained therein are accurate and reflect the most up-to-date evidence available at time of publication. All our reports are peer- reviewed for accuracy and completeness by external scientists with expertise in ecology, fisheries science or aquaculture. Scientific review, however, does not constitute an endorsement of the Seafood Watch program or its recommendations on the part of the reviewing scientists. Seafood Watch is solely responsible for the conclusions reached in this report. We always welcome additional or updated data that can be used for the next revision. Seafood Watch and Seafood Reports are made possible through a grant from the David and Lucile Packard Foundation. 2 Final Seafood Recommendation This report covers wild-caught California spiny lobster caught by traps in California waters. This species is a Good Alternative. Impacts Impacts on Manage- Habitat and Stock Fishery on the Overall other Species ment Ecosystem Stock Rank Lowest scoring species Rank Rank Recommendation (Score) Rank*, Subscore, Score Score Score Score California Spiny Lobster California Spiny California Spiny Lobster Yellow Yellow Yellow GOOD ALTERNATIVE Lobster, Cormorants 3.05 3 3.12 2.84 Yellow, 3.05,2.29 Scoring note – scores range from zero to five where zero indicates very poor performance and five indicates -

Msc 2Nd Re-Assessment Public Client Draft Report
SCS Global Services Report MSC 2ND RE-ASSESSMENT PUBLIC CLIENT DRAFT REPORT Mexico Baja California red rock lobster fishery Prepared for: Federación Regional de Sociedades Cooperativas de la Industria Pesquera de Baja California, F.C.L. (FEDECOOP) Date of Field Audit: November 17-18, 2015 Report Delivered: July 29, 2016 Prepared by: Dr. Carlos Alvarez Flores, Stock Assessment Consultant, Team Leader/Principle 1 and 3 Expert Ms. Sandra Andraka, Fisheries Consultant, Principle 2 Expert Dr. Sian Morgan, Procedural oversight Ms. Gabriela Anhalzer, Coordination Natural Resources Division +1.510.452.6392 [email protected] 2000 Powell Street, Ste. 600, Emeryville, CA 94608 USA +1.510.452.8000 main | +1.510-452-8001 fax www.SCSGlobalServices.com SCSglobalservices.com Table of Contents MSC 2ND RE-ASSESSMENT – PUBLIC CLIENT DRAFT REPORT ......................................................... 1 Table of Contents ..................................................................................................................... i Glossary ................................................................................................................................. 4 1. Executive Summary .......................................................................................................... 7 2. Authorship and Peer Reviewers ...................................................................................... 10 Audit Team ...................................................................................................................