Urinary Incontinence
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CMS Manual System Human Services (DHHS) Pub
Department of Health & CMS Manual System Human Services (DHHS) Pub. 100-07 State Operations Centers for Medicare & Provider Certification Medicaid Services (CMS) Transmittal 8 Date: JUNE 28, 2005 NOTE: Transmittal 7, of the State Operations Manual, Pub. 100-07 dated June 27, 2005, has been rescinded and replaced with Transmittal 8, dated June 28, 2005. The word “wound” was misspelled in the Interpretive Guidance section. All other material in this instruction remains the same. SUBJECT: Revision of Appendix PP – Section 483.25(d) – Urinary Incontinence, Tags F315 and F316 I. SUMMARY OF CHANGES: Current Guidance to Surveyors is entirely replaced by the attached revision. The two tags are being combined as one, which will become F315. Tag F316 will be deleted. The regulatory text for both tags will be combined, followed by this revised guidance. NEW/REVISED MATERIAL - EFFECTIVE DATE*: June 28, 2005 IMPLEMENTATION DATE: June 28, 2005 Disclaimer for manual changes only: The revision date and transmittal number apply to the red italicized material only. Any other material was previously published and remains unchanged. However, if this revision contains a table of contents, you will receive the new/revised information only, and not the entire table of contents. II. CHANGES IN MANUAL INSTRUCTIONS: (N/A if manual not updated.) (R = REVISED, N = NEW, D = DELETED) – (Only One Per Row.) R/N/D CHAPTER/SECTION/SUBSECTION/TITLE R Appendix PP/Tag F315/Guidance to Surveyors – Urinary Incontinence D Appendix PP/Tag F316/Urinary Incontinence III. FUNDING: Medicare contractors shall implement these instructions within their current operating budgets. IV. ATTACHMENTS: Business Requirements x Manual Instruction Confidential Requirements One-Time Notification Recurring Update Notification *Unless otherwise specified, the effective date is the date of service. -

Urology Services in the ASC
Urology Services in the ASC Brad D. Lerner, MD, FACS, CASC Medical Director Summit ASC President of Chesapeake Urology Associates Chief of Urology Union Memorial Hospital Urologic Consultant NFL Baltimore Ravens Learning Objectives: Describe the numerous basic and advanced urology cases/lines of service that can be provided in an ASC setting Discuss various opportunities regarding clinical, operational and financial aspects of urology lines of service in an ASC setting Why Offer Urology Services in Your ASC? Majority of urologic surgical services are already outpatient Many urologic procedures are high volume, short duration and low cost Increasing emphasis on movement of site of service for surgical cases from hospitals and insurance carriers to ASCs There are still some case types where patients are traditionally admitted or placed in extended recovery status that can be converted to strictly outpatient status and would be suitable for an ASC Potential core of fee-for-service case types (microsurgery, aesthetics, prosthetics, etc.) Increasing Population of Those Aged 65 and Over As of 2018, it was estimated that there were 51 million persons aged 65 and over (15.63% of total population) By 2030, it is expected that there will be 72.1 million persons aged 65 and over National ASC Statistics - 2017 Urology cases represented 6% of total case mix for ASCs Urology cases were 4th in median net revenue per case (approximately $2,400) – behind Orthopedics, ENT and Podiatry Urology comprised 3% of single specialty ASCs (5th behind -

Urinary Tract Infection (UTI): Western and Ayurvedic Diagnosis and Treatment Approaches
Urinary tract infection (UTI): Western and Ayurvedic Diagnosis and Treatment Approaches. By: Mahsa Ranjbarian Urinary system Renal or Urinary system is one of the 10 body systems that we have. This system is the body drainage system. The urinary system is composed of kidneys (vrikka), ureters (mutravaha nadis), bladder(mutrashaya) and urethra(mutramarga). The kidneys are a pair of bean-shaped, fist size organs that lie in the middle of the back, just below the rib cage, one on each side of the spine. Ureters are tubes that carry the wastes or urine from the kidneys to the bladder. The urine finally exit the body from the urethra when the bladder is full.1 Urethras length is shorter in women than men due to the anatomical differences. Major function of the urinary system is to remove wastes and water from our body through urination. Other important functions of the urinary system are as follows. 1. Prevent dehydration and at the same time prevent the buildup of extra fluid in the body 2. Cleans the blood of metabolic wastes 3. Removing toxins from the body 4. Maintaining the homeostasis of many factors including blood PH and blood pressure 5. Producing erythrocytes 6. make hormones that help regulate blood pressure 7. keep bones strong 8. keep levels of electrolytes, such as potassium and phosphate, stable 2 The Urinary system like any other systems of our body is working under the forces of three doshas, subdoshas. Mutravaha srotas, Ambuvahasrota and raktavahasrota are involved in formation and elimination of the urine. Urine gets separated from the rasa by maladhara kala with the help of pachaka pitta and samana vayu and then through the mutravaha srota(channels carrying the urine) it is taken to the bladder. -

Urinary Tract Infection (Uti) Fact Sheet
URINARY TRACT INFECTION (UTI) FACT SHEET Overview A urinary tract infection (UTI) is an infection that involves any part of the urinary tract, including the kidneys, bladder and urethra. It is usually caused by exposure of the urinary tract to a fecal organism such as Escherichia coli (E. coli) but also may be caused by other organisms. An indwelling urinary catheter is a drainage tube that is inserted into the urinary bladder through the urethra, is left in place and is connected to a closed urine collection system. Urinary tract infections in patients with an indwelling urinary catheter are called catheter-associated UTIs, or CAUTIs. Signs and Symptoms Common symptoms of UTI include: • fever; • pain or burning in the lower abdomen; • burning during urination; • an increase in the frequency of urination; and • cloudy appearing urine. Causes and Transmission A UTI occurs when germs (usually bacteria) enter the urinary tract through the meatus (the opening of the urinary tract). These germs then cause infection. The urinary tract is normally sterile, meaning it contains no germs. A CAUTI occurs when germs (usually bacteria) enter the urinary tract through the urinary catheter and cause infection. This can occur when health care worker hands are not properly cleaned before the insertion of a catheter or during the process of cleaning or emptying of the urine collection system. Risk Factors Sexually active women, people with blockages in the urinary tract, such as prostate enlargement, and women using certain types of birth control, such as diaphragms, or those who are undergoing menopause are at higher risk of UTI. -

233 the Use of Absorbent Pads Increases the Risk For
233 Omli R1, Skotnes L H1, Romild U1, Kuhry E1 1. Namsos Hospital THE USE OF ABSORBENT PADS INCREASES THE RISK FOR LOWER URINARY TRACT INFECTIONS IN NURSING HOMES RESIDENTS. Hypothesis / aims of study Urinary incontinence is a common medical problem in the nursing home population. The aim of this study was to determine whether use of absorbent pads is a risk factor for the development of lower urinary tract infections in nursing home residents. Introduction Lower urinary tract infections (UTIs) are a common medical problem in the elderly. Different kinds of absorbent products, such as diapers, pad and pant combinations, are used in incontinence care. In some cases, indwelling catheters, external condom catheters or in-and-out catheterisation are options for managing bladder dysfunction. About 23% of the residents in nursing homes don’t need toilet assistance. That means that the majority of residents is dependent on help from nursing staff for toileting and help to change soiled absorbent pads. Transmission of bacteria during incontinence care is associated with UTIs in elderly. In the vulnerable nursing home population, UTIs are a frequent problem and can lead to increased morbidity and even mortality. The prevalence of UTIs in nursing home residents is approximately 30%. Improving hand hygiene among staffing in nursing homes is one of the most important and effective methods to reduce hand born transmission of pathological microbes. Studies in long-term-care facilities document a lack of hand hygiene in connection to staff-resident interactions. This study studies the association between the use of absorbent pads and symptomatic UTIs in the nursing home population. -
Module 3: Benign Prostatic Hypertrophy
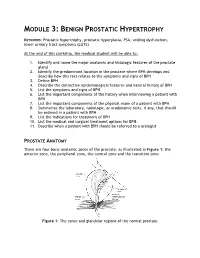
MODULE 3: BENIGN PROSTATIC HYPERTROPHY KEYWORDS: Prostatic hypertrophy, prostatic hyperplasia, PSA, voiding dysfunction, lower urinary tract symptoms (LUTS) At the end of this clerkship, the medical student will be able to: 1. Identify and name the major anatomic and histologic features of the prostate gland 2. Identify the predominant location in the prostate where BPH develops and describe how this fact relates to the symptoms and signs of BPH 3. Define BPH 4. Describe the distinctive epidemiological features and natural history of BPH 5. List the symptoms and signs of BPH 6. List the important components of the history when interviewing a patient with BPH 7. List the important components of the physical exam of a patient with BPH 8. Summarize the laboratory, radiologic, or urodynamic tests, if any, that should be ordered in a patient with BPH 9. List the indications for treatment of BPH 10. List the medical and surgical treatment options for BPH. 11. Describe when a patient with BPH should be referred to a urologist PROSTATE ANATOMY There are four basic anatomic zones of the prostate, as illustrated in Figure 1: the anterior zone, the peripheral zone, the central zone and the transition zone. Figure 1: The zones and glandular regions of the normal prostate. The anterior zone is entirely fibromuscular and non-glandular, and it appears to have little significance in prostatic function or pathology. This area comprises approximately 20% of the bulk of prostatic tissue. The peripheral zone is composed entirely of acinar tissue. It comprises the posterior surface of the prostate, including the apical, lateral, posterolateral and anterolateral portions of the prostate. -

Urinary Incontinence ! ! !What Is Urinary Incontinence? Leakage of Urine Is Called Urinary Incontinence
! ! ! Urinary Incontinence ! ! !What is urinary incontinence? Leakage of urine is called urinary incontinence. Some women leak small amounts of !urine. At other times, leakage of urine is frequent or severe. !Are there different types of urinary incontinence? !There are several types of urinary incontinence: • Stress urinary incontinence is loss of urine when a woman coughs, laughs, or sneezes. Leaks also can happen when a woman walks, runs, or exercises. It is caused by a weakening of the tissues that support the bladder or the muscles of the ! urethra. • Urge incontinence is leakage of urine caused by overactive bladder muscles that ! contract too often or problems with the nerves that send signals to the bladder !• Mixed incontinence is a combination of both stress and urge incontinence symptoms • Overflow incontinence is a steady loss of small amounts of urine when the bladder does not empty all the way during voiding. It can be caused by an underactive bladder ! muscle or blockage of the urethra. !What are the symptoms of urinary incontinence? !In addition to leaking urine, a woman with incontinence also may have other symptoms: • Urgency— A strong urge to urinate whether or not the bladder is full, often with pelvic ! pressure !• Frequency— Voiding more often than she considers usual !• Nocturia — The need to void during hours of sleep !• Dysuria — Painful voiding !• Enuresis— Bed-wetting or leaking while sleeping ! What causes urinary incontinence? ! Urinary incontience can have short-term causes and long-term causes. Short-term !causes are easier to treat and include the following: • Urinary tract infection— Loss of bladder control may be caused by an infection of the urinary tract. -

Frequently Asked Questions About Overactive Bladder
ABOUT OAB Frequently Asked Questions about Overactive Bladder What is Overactive Bladder (OAB)? If you live with OAB, you may also: Overactive Bladder (OAB) isn’t a disease. It’s the u Leak urine (incontinence): Sometimes people name of a group of urinary symptoms. The most with OAB also have “urgency incontinence.” common symptom of OAB is a sudden urge to This means that urine leaks when you feel urinate that you can’t control. Some people will the sudden urge to go. This isn’t the same as leak urine when they feel this urge. Having to “stress urinary incontinence” or “SUI.” People urinate many times during the day and night is with SUI leak urine while sneezing, laughing or another symptom of OAB. doing other physical activities. (You can learn more about SUI at UrologyHealth.org/SUI.) How common is OAB? u Urinate frequently: You may also need to go OAB is common. It affects millions of Americans. to the bathroom many times during the day. As many as 30 percent of men and 40 percent The number of times someone urinates varies of women in the United States live with OAB from person to person. But many experts symptoms. agree that going to the bathroom more than eight times in 24 hours is “frequent urination.” Who is at risk for OAB? u Wake up at night to urinate: Waking from As you grow older, you’re at higher risk for sleep to go to the bathroom more than once a OAB. But no matter what your age, there are night is another symptom of OAB. -

Guide to Treating Your Child's Daytime Or Nighttime Accidents
A GUIDE TO TREATING YOUR CHILD’S Daytime or Nighttime Accidents, Urinary Tract Infections and Constipation UCSF BENIOFF CHILDREN’S HOSPITALS UROLOGY DEPARTMENT This booklet contains information that will help you understand more about your child’s bladder problem(s) and provides tips you can use at home before your first visit to the urology clinic. www.childrenshospitaloakland.org | www.ucsfbenioffchildrens.org 2 | UCSF BENIOFF CHILDREN’S HOSPITALS UROLOGY DEPARTMENT Table of Contents Dear Parent(s), Your child has been referred to the Pediatric Urology Parent Program at UCSF Benioff Children’s Hospitals. We specialize in the treatment of children with bladder and bowel dysfunction. This booklet contains information that will help you understand more about your child’s problem(s) and tips you can use at home before your first visit to the urology clinic. Please review the sections below that match your child’s symptoms. 1. Stool Retention and Urologic Problems (p.3) (Bowel Dysfunction) 2. Bladder Dysfunction (p.7) Includes daytime incontinence (wetting), urinary frequency and infrequency, dysuria (painful urination) and overactive bladder 3. Urinary Tract Infection and Vesicoureteral Reflux (p. 10) 4. Nocturnal Enuresis (p.12) Introduction (Nighttime Bedwetting) It’s distressing to see your child continually having accidents. The good news is that the problem is very 5. Urologic Tests (p.15) common – even if it doesn’t feel that way – and that children generally outgrow it. However, the various interventions we offer can help resolve the issue sooner THIS BOOKLET ALSO CONTAINS: rather than later. » Resources for Parents (p.16) » References (p.17) Childhood bladder and bowel dysfunction takes several forms. -

60 Seasonal Variation in Urinary Frequency
60 Cartwright R1, Rajan P2, Swamy S3, Mariappan P4, Turner K J5, Stewart L4, Khullar V1 1. Dept of Urogynaecology, St Mary's Hospital, London, UK, 2. Dept of Urology, Glasgow Royal Infirmary, UK, 3. Dept of Urogynaecology, St Thomas' Hospital, London, UK, 4. Dept of Urology, Western General Hospital, Edinburgh, UK, 5. Dept of Urology, Royal Bournemouth Hospital, UK SEASONAL VARIATION IN URINARY FREQUENCY, NOCTURIA, AND OTHER LOWER URINARY TRACT SYMPTOMS IN MEN AND WOMEN Hypothesis / aims of study At a population level there are small seasonal effects on the prevalence of clinically relevant lower urinary tract storage symptoms [1], and a significant interaction with overactive bladder treatment response [2]. However, the effect of temperature variation on clinical assessment of lower urinary tract symptoms has not been explored. The aim of this study was to assess the association between atmospheric temperature, bladder diary variables, symptom severity, and urodynamic parameters in men and women with lower urinary tract symptoms. Study design, materials and methods Data were collected at two urodynamic units, Centre 1 situated at 55°52′N, equivalent to Moscow, and Centre 2 at 51°30′N, equivalent to Rotterdam, both with maritime temperate climates (Köppen classification Cfb). Consecutive men and women referred for evaluation were asked to complete both a 3 day frequency-volume chart, and a standardised lower urinary tract symptom and quality of life questionnaire (International Prostate Symptoms Score for men, and King’s Health Questionnaire for women), before undergoing free uroflowmetry, and where indicated, multichannel saline cystometry. Local mean monthly temperatures for each centre were extracted from national meteorological records. -

EAU Guidelines on Urinary Incontinence in Adults
EAU Guidelines on Urinary Incontinence in Adults F.C . Burkhard (Chair), J.L.H.R. Bosch, F. Cruz, G.E. Lemack, A.K. Nambiar, N. Thiruchelvam, A. Tubaro Guidelines Associates: D. Ambühl, D.A. Bedretdinova, F. Farag, R. Lombardo, M.P. Schneider © European Association of Urology 2018 TABLE OF CONTENTS PAGE 1. INTRODUCTION 8 1.1 Aim and objectives 8 1.1.1 The elderly 8 1.2 Panel composition 8 1.3 Available publications 8 1.4 Publication history 9 1.4.1 Summary of changes. 9 2. METHODS 11 2.1 Introduction 11 2.2 Review 11 2.3 Future goals 11 3. DIAGNOSTIC EVALUATION 11 3.1 History and physical examination 11 3.2 Patient questionnaires 12 3.2.1 Questions 12 3.2.2 Evidence 12 3.2.3 Summary of evidence and recommendations for patient questionnaires 13 3.3 Voiding diaries 14 3.3.1 Question 14 3.3.2 Evidence 14 3.3.3 Summary of evidence and recommendations for voiding diaries 14 3.4 Urinalysis and urinary tract infection 14 3.4.1 Question 14 3.4.2 Evidence 14 3.4.3 Summary of evidence and recommendations for urinalysis 15 3.5 Post-void residual volume 15 3.5.1 Question 15 3.5.2 Evidence 15 3.5.3 Summary of evidence and recommendations for post-void residual 15 3.6 Urodynamics 15 3.6.1 Question 16 3.6.2 Evidence 16 3.6.2.1 Variability 16 3.6.2.2 Diagnostic accuracy 16 3.6.2.3 Question 16 3.6.2.4 Evidence 16 3.6.2.5 Question 16 3.6.2.6 Evidence 16 3.6.2.7 Question 17 3.6.2.8 Evidence 17 3.6.2.9 Question 17 3.6.2.10 Evidence 17 3.6.3 Summary of evidence and recommendations for urodynamics 17 3.6.4 Research priority 18 3.7 Pad testing 18 3.7.1 Questions 18 3.7.2 Evidence 18 3.7.3 Summary of evidence and recommendations for pad testing 18 3.7.4 Research priority 18 3.8 Imaging 18 3.8.1 Questions 19 3.8.2 Evidence 19 3.8.3 Summary of evidence and recommendations for imaging 19 3.8.4 Research priority 19 2 URINARY INCONTINENCE IN ADULTS - LIMITED UPDATE MARCH 2018 4. -

Invasive Treatments for Urinary Incontinence
Cigna Medical Coverage Policy Effective Date .......................... 12/15/2013 Subject Invasive Treatments for Next Review Date .................... 12/15/2014 Coverage Policy Number ................. 0365 Urinary Incontinence Table of Contents Hyperlink to Related Coverage Policies Coverage Policy .................................................. 1 Biofeedback General Background ........................................... 2 Botulinum Therapy Coding/Billing Information ................................. 15 Electrical Stimulators References ........................................................ 16 Extracorporeal Electromagnetic Stimulation for Urinary Incontinence Injectable Bulking Agents for Urinary Conditions and Fecal Incontinence Physical Therapy Sacral Nerve Stimulation for Urinary Voiding Dysfunction and Fecal Incontinence INSTRUCTIONS FOR USE The following Coverage Policy applies to health benefit plans administered by Cigna companies. Coverage Policies are intended to provide guidance in interpreting certain standard Cigna benefit plans. Please note, the terms of a customer’s particular benefit plan document [Group Service Agreement, Evidence of Coverage, Certificate of Coverage, Summary Plan Description (SPD) or similar plan document] may differ significantly from the standard benefit plans upon which these Coverage Policies are based. For example, a customer’s benefit plan document may contain a specific exclusion related to a topic addressed in a Coverage Policy. In the event of a conflict, a customer’s benefit plan document