Ribonucleotides
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Philosophy of Science and Philosophy of Chemistry
Philosophy of Science and Philosophy of Chemistry Jaap van Brakel Abstract: In this paper I assess the relation between philosophy of chemistry and (general) philosophy of science, focusing on those themes in the philoso- phy of chemistry that may bring about major revisions or extensions of cur- rent philosophy of science. Three themes can claim to make a unique contri- bution to philosophy of science: first, the variety of materials in the (natural and artificial) world; second, extending the world by making new stuff; and, third, specific features of the relations between chemistry and physics. Keywords : philosophy of science, philosophy of chemistry, interdiscourse relations, making stuff, variety of substances . 1. Introduction Chemistry is unique and distinguishes itself from all other sciences, with respect to three broad issues: • A (variety of) stuff perspective, requiring conceptual analysis of the notion of stuff or material (Sections 4 and 5). • A making stuff perspective: the transformation of stuff by chemical reaction or phase transition (Section 6). • The pivotal role of the relations between chemistry and physics in connection with the question how everything fits together (Section 7). All themes in the philosophy of chemistry can be classified in one of these three clusters or make contributions to general philosophy of science that, as yet , are not particularly different from similar contributions from other sci- ences (Section 3). I do not exclude the possibility of there being more than three clusters of philosophical issues unique to philosophy of chemistry, but I am not aware of any as yet. Moreover, highlighting the issues discussed in Sections 5-7 does not mean that issues reviewed in Section 3 are less im- portant in revising the philosophy of science. -

Alternative Biochemistries for Alien Life: Basic Concepts and Requirements for the Design of a Robust Biocontainment System in Genetic Isolation
G C A T T A C G G C A T genes Review Alternative Biochemistries for Alien Life: Basic Concepts and Requirements for the Design of a Robust Biocontainment System in Genetic Isolation Christian Diwo 1 and Nediljko Budisa 1,2,* 1 Institut für Chemie, Technische Universität Berlin Müller-Breslau-Straße 10, 10623 Berlin, Germany; [email protected] 2 Department of Chemistry, University of Manitoba, 144 Dysart Rd, 360 Parker Building, Winnipeg, MB R3T 2N2, Canada * Correspondence: [email protected] or [email protected]; Tel.: +49-30-314-28821 or +1-204-474-9178 Received: 27 November 2018; Accepted: 21 December 2018; Published: 28 December 2018 Abstract: The universal genetic code, which is the foundation of cellular organization for almost all organisms, has fostered the exchange of genetic information from very different paths of evolution. The result of this communication network of potentially beneficial traits can be observed as modern biodiversity. Today, the genetic modification techniques of synthetic biology allow for the design of specialized organisms and their employment as tools, creating an artificial biodiversity based on the same universal genetic code. As there is no natural barrier towards the proliferation of genetic information which confers an advantage for a certain species, the naturally evolved genetic pool could be irreversibly altered if modified genetic information is exchanged. We argue that an alien genetic code which is incompatible with nature is likely to assure the inhibition of all mechanisms of genetic information transfer in an open environment. The two conceivable routes to synthetic life are either de novo cellular design or the successive alienation of a complex biological organism through laboratory evolution. -

Peptide Chemistry up to Its Present State
Appendix In this Appendix biographical sketches are compiled of many scientists who have made notable contributions to the development of peptide chemistry up to its present state. We have tried to consider names mainly connected with important events during the earlier periods of peptide history, but could not include all authors mentioned in the text of this book. This is particularly true for the more recent decades when the number of peptide chemists and biologists increased to such an extent that their enumeration would have gone beyond the scope of this Appendix. 250 Appendix Plate 8. Emil Abderhalden (1877-1950), Photo Plate 9. S. Akabori Leopoldina, Halle J Plate 10. Ernst Bayer Plate 11. Karel Blaha (1926-1988) Appendix 251 Plate 12. Max Brenner Plate 13. Hans Brockmann (1903-1988) Plate 14. Victor Bruckner (1900- 1980) Plate 15. Pehr V. Edman (1916- 1977) 252 Appendix Plate 16. Lyman C. Craig (1906-1974) Plate 17. Vittorio Erspamer Plate 18. Joseph S. Fruton, Biochemist and Historian Appendix 253 Plate 19. Rolf Geiger (1923-1988) Plate 20. Wolfgang Konig Plate 21. Dorothy Hodgkins Plate. 22. Franz Hofmeister (1850-1922), (Fischer, biograph. Lexikon) 254 Appendix Plate 23. The picture shows the late Professor 1.E. Jorpes (r.j and Professor V. Mutt during their favorite pastime in the archipelago on the Baltic near Stockholm Plate 24. Ephraim Katchalski (Katzir) Plate 25. Abraham Patchornik Appendix 255 Plate 26. P.G. Katsoyannis Plate 27. George W. Kenner (1922-1978) Plate 28. Edger Lederer (1908- 1988) Plate 29. Hennann Leuchs (1879-1945) 256 Appendix Plate 30. Choh Hao Li (1913-1987) Plate 31. -

Geochemical Influences on Nonenzymatic Oligomerization Of
bioRxiv preprint doi: https://doi.org/10.1101/872234; this version posted December 11, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Geochemical influences on nonenzymatic oligomerization of prebiotically relevant cyclic nucleotides Authors: Shikha Dagar‡, Susovan Sarkar‡, Sudha Rajamani‡* ‡ Department of Biology, Indian Institute of Science Education and Research, Pune 411008, India Correspondence: [email protected]; Tel.: +91-20-2590-8061 Running title: Cyclic nucleotides and emergence of an RNA World Key words: Dehydration-rehydration cycles, lipid-assisted oligomerization, cyclic nucleotides, analogue environments Dagar, S. 1 bioRxiv preprint doi: https://doi.org/10.1101/872234; this version posted December 11, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract The spontaneous emergence of RNA on the early Earth continues to remain an enigma in the field of origins of life. Few studies have looked at the nonenzymatic oligomerization of cyclic nucleotides under neutral to alkaline conditions, in fully dehydrated state. Herein, we systematically investigated the oligomerization of cyclic nucleotides under prebiotically relevant conditions, where starting reactants were subjected to repeated dehydration-rehydration (DH- RH) regimes, like they would have been on an early Earth. DH-RH conditions, a recurring geological theme, are driven by naturally occurring processes including diurnal cycles and tidal pool activity. These conditions have been shown to facilitate uphill oligomerization reactions in terrestrial geothermal niches, which are hypothesized to be pertinent sites for the emergence of life. -

Lecture 1: Strategies and Tactics in Organic Synthesis
Massachusetts Institute of Technology Organic Chemistry 5.511 October 3, 2007 Prof. Rick L. Danheiser Lecture 1: Strategies and Tactics in Organic Synthesis Retrosynthetic Analysis The key to the design of efficient syntheses ". the grand thing is to be able to reason backwards. That is a very useful accomplishment, and a very easy "The end is where we start from...." one, but people do not practice it much." T. S. Eliot, in "The Four Quartets" Sherlock Holmes, in "A Study in Scarlet" Strategy Tactics overall plan to achieve the means by which plan ultimate synthetic target is implemented intellectual experimental retrosynthetic planning synthetic execution TRANSFORMS REACTIONS Target Precursor Precursor Target Definitions Retron Structural unit that signals the application of a particular strategy algorithm during retrosynthetic analysis. Transform Imaginary retrosynthetic operation transforming a target molecule into a precursor molecule in a manner such that bond(s) can be reformed (or cleaved) by known or reasonable synthetic reactions. Strategy Algorithm Step-by-step instructions for performing a retrosynthetic operation. "...even in the earliest stages of the process of simplification of a synthetic problem, the chemist must make use of a particular form of analysis which depends on the interplay between structural features that exist in the target molecule and the types of reactions or synthetic operations available from organic chemistry for the modification or assemblage of structural units. The synthetic chemist has learned by experience to recognize within a target molecule certain units which can be synthesized, modified, or joined by known or conceivable synthetic operations...it is convenient to have a term for such units; the term "synthon" is suggested. -
![[2.2]Paracyclophanes- Structure and Reactivity Studies Von Der](https://docslib.b-cdn.net/cover/2287/2-2-paracyclophanes-structure-and-reactivity-studies-von-der-1602287.webp)
[2.2]Paracyclophanes- Structure and Reactivity Studies Von Der
Syntheses of Functionalised [2.2]Paracyclophanes- Structure and Reactivity Studies Von der Gemeinsamen Naturwissenschaftlichen Fakultät der Technischen Universität Carolo Wilhelmina zu Braunschweig zur Erlangung des Grades eines Doktors der Naturwissenschaften (Dr.rer.nat.) genehmigte D i s s e r t a t i o n von Swaminathan Vijay Narayanan aus Chennai (Madras) / India 1. Referent: Prof. Dr. Dr. h. c. Henning Hopf 2. Referentin: Prof. Dr. Monika Mazik eingereicht am: 20. Jan 2005 mündliche Prüfung (Disputation) am: 29. März 2005 Druckjahr 2005 Vorveröffentlichungen der Dissertation Teilergebnisse aus dieser Arbeit wurden mit Genehmigung der Gemeinsamen Naturwissenschaft-lichen Fakultät, vertreten durch die Mentorin oder den Mentor/die Betreuerin oder den Betreuer der Arbeit, in folgenden Beiträgen vorab veröffentlicht: Publikationen K. El Shaieb, V. Narayanan, H. Hopf, I. Dix, A. Fischer, P. G. Jones, L. Ernst & K. Ibrom.: 4,15-Diamino[2.2]paracyclophane as a starting material for pseudo-geminally substituted [2.2]paracyclophanes. Eur. J. Org. Chem.: 567-577 (2003). Die vorliegende Arbeit wurde in der Zeit von Oktober 2001 bis Januar 2004 am Institut für Organische Chemie der Technischen Universität Braunschweig unter der Leitung von Prof. Dr. Dr. h.c. Henning Hopf angefertigt. It is my great pleasure to express my sincere gratitude to Prof. Dr. Henning Hopf for his support, encouragement and guidance throughout this research work. I thank him a lot for his invaluable ideas and remarks which made this study very interesting. I admire deep from my heart his energetic way of working and his brilliant ideas which made possible this research work to cover a vast area. -
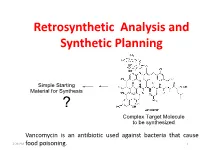
Retrosynthetic Analysis and Synthetic Planning
Retrosynthetic Analysis and Synthetic Planning Vancomycin is an antibiotic used against bacteria that cause 2:24 PM food poisoning. 1 Life’s Perspectives Planning a Journey to the Unknown 2:24 PM 2 Retrosynthetic Analysis Definition Retrosynthetic analysis (retrosynthesis) is a technique for planning a synthesis, especially of complex organic molecules, whereby the complex target molecule (TM) is reduced into a sequence of progressively simpler structures along a pathway which ultimately leads to the identification of a simple or commercially available starting material (SM) from which a chemical synthesis can then be developed. Retrosynthetic analysis is based on known reactions (e.g the Wittig reaction, oxidation, reduction etc). The synthetic plan generated from the retrosynthetic analysis will be the roadmap to guide the synthesis of the target molecule. 2:24 PM 3 Synthetic Planning Definition Synthesis is a construction process that involves converting simple or commercially available molecules into complex molecules using specific reagents associated with known reactions in the retrosynthetic scheme. Syntheses can be grouped into two broad categories: (i) Linear syntheses 2:24 PM (ii)Convergent syntheses 4 Linear Synthesis Definition In linear synthesis, the target molecule is synthesized through a series of linear transformations. Since the overall yield of the synthesis is based on the single longest route to the target molecule, by being long, a linear synthesis suffers a lower overall yield. The linear synthesis is fraught with failure for its lack of flexibility leading to potential large losses in the material already invested in the synthesis at the time of failure. 2:24 PM 5 Convergent Synthesis Definition In convergent synthesis, key fragments of the target molecule are synthesized separately or independently and then brought together at a later stage in the synthesis to make the target molecule. -

Nucleobases Thin Films Deposited on Nanostructured Transparent Conductive Electrodes for Optoelectronic Applications
www.nature.com/scientificreports OPEN Nucleobases thin flms deposited on nanostructured transparent conductive electrodes for optoelectronic applications C. Breazu1*, M. Socol1, N. Preda1, O. Rasoga1, A. Costas1, G. Socol2, G. Petre1,3 & A. Stanculescu1* Environmentally-friendly bio-organic materials have become the centre of recent developments in organic electronics, while a suitable interfacial modifcation is a prerequisite for future applications. In the context of researches on low cost and biodegradable resource for optoelectronics applications, the infuence of a 2D nanostructured transparent conductive electrode on the morphological, structural, optical and electrical properties of nucleobases (adenine, guanine, cytosine, thymine and uracil) thin flms obtained by thermal evaporation was analysed. The 2D array of nanostructures has been developed in a polymeric layer on glass substrate using a high throughput and low cost technique, UV-Nanoimprint Lithography. The indium tin oxide electrode was grown on both nanostructured and fat substrate and the properties of the heterostructures built on these two types of electrodes were analysed by comparison. We report that the organic-electrode interface modifcation by nano- patterning afects both the optical (transmission and emission) properties by multiple refections on the walls of nanostructures and the electrical properties by the efect on the organic/electrode contact area and charge carrier pathway through electrodes. These results encourage the potential application of the nucleobases thin flms deposited on nanostructured conductive electrode in green optoelectronic devices. Te use of natural or nature-inspired materials in organic electronics is a dynamic emerging research feld which aims to replace the synthesized materials with natural (bio) ones in organic electronics1–3. -

Nucleobase-Containing Compounds Evoke Behavioural, Olfactory, and Transcriptional Responses in Model Fishes
Correction-Nucleobase-containing compounds evoke behavioural, olfactory, and transcriptional responses in model fishes Shamchuk, A. L., Blunt, B. J., Lyons, D. D., Wang, M. Q., Gasheva, A., Lewis, C. R., Tomlin, K., Starr Hazard, E., Hardiman, G., & Tierney, K. B. (2018). Correction-Nucleobase-containing compounds evoke behavioural, olfactory, and transcriptional responses in model fishes. FACETS, 3(1). https://doi.org/10.1139/facets-2017-0101 Published in: FACETS Document Version: Publisher's PDF, also known as Version of record Queen's University Belfast - Research Portal: Link to publication record in Queen's University Belfast Research Portal Publisher rights Copyright 2018 the authors. This is an open access article published under a Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided the author and source are cited. 4.0), which permits unrestricted use, General rights Copyright for the publications made accessible via the Queen's University Belfast Research Portal is retained by the author(s) and / or other copyright owners and it is a condition of accessing these publications that users recognise and abide by the legal requirements associated with these rights. Take down policy The Research Portal is Queen's institutional repository that provides access to Queen's research output. Every effort has been made to ensure that content in the Research Portal does not infringe any person's rights, or applicable UK laws. If you discover content in the Research Portal that you believe breaches copyright or violates any law, please contact [email protected]. -

Development of a Universal Nucleobase and Modified
Development of a Universal Nucleobase and UNIT 1.5 Modified Nucleobases for Expanding the Genetic Code This unit presents protocols for the synthesis and characterization of nucleosides with unnatural bases in order to develop bases for the expansion of the genetic alphabet or for nonselective pairing opposite natural bases. The faithful pairing of nucleobases through complementary hydrogen-bond (H-bond) donors and acceptors forms the foundation of the genetic code. However, there is no reason to assume that the requirements for duplex stability and replication must limit the genetic alphabet to only two base pairs, or, for that matter, hydrogen-bonded base pairs. Expansion of this alphabet to contain a third base pair would allow for the encoding of additional information and would make possible a variety of in vitro experiments using nucleic acids with unnatural building blocks. Previous efforts to generate orthogonal base pairs have relied on H-bonding patterns that are not found with the canonical Watson-Crick pairs. However, in all cases, the unnatural bases were not kinetically orthogonal, and instead competitively paired with natural bases during polymerase-catalyzed DNA synthesis (Horlacher et al., 1995; Lutz et al., 1996, 1998a,b). Tautomeric isomerism, which would alter H-bond donor and acceptor patterns, likely contributes to this kinetic infidelity (Roberts et al., 1997a,b; Robinson et al., 1998; Beaussire and Pochet, 1999). An alternative strategy is centered around developing unnatural bases that form pairs based not on hydrogen bonds, but rather on interbase hydrophobic interactions. Such hydrophobic bases should not pair stably opposite natural bases due to the forced desolvation of the purines or pyrimidines. -

12.1 One-Step Syntheses
12.1 One‐Step Syntheses 12.1 One‐Step Syntheses • When building a HOME, you must have the right tools, • You may be able to solve one‐step syntheses by but you also need to know HOW to use the tools. matching the reaction pattern with an example in the • When building a MOLECULE (designing a synthesis), a textbook. lot of tools are available. Where are they? – To add Br–Br across a C=C double bond, an alkene is generally treated with Br / CCl in the dark. • To optimize your synthesis, you must understand HOW 2 4 the tools work. WHY? • How do you solve such syntheses without the textbook and without having to memorize each set of reagents? • What tools (reagents and conditions) are needed for – Learning to use any tool requires PRACTICE. the synthesis below? – Its also helpful to know mechanisms. WHY? Copyright 2012 John Wiley & Sons, Inc. 12 -1 Klein, Organic Chemistry 1e Copyright 2012 John Wiley & Sons, Inc. 12 -2 Klein, Organic Chemistry 1e 12.2 Functional Group 12.1 One‐Step Syntheses Transformations • Solving one‐step syntheses: • Let’s review some synthetic tools we have learned so 1. Analyze the structures of the reactant and product. far. 2. Assess HOW the functional groups have changed. • In Chapter 9, we learned how to shift the position of a 3. Use the reactions and mechanisms you have learned to halide. determine appropriate reagents and conditions. 4. Check your answer by working out the mechanism. • Solve the synthesis. • The regiochemical considerations in this process are • Practice with CONCEPTUAL CHECKPOINTs vital to making the correct product. -

Overview of the Synthesis of Nucleoside Phosphates and Polyphosphates 13.1.6
Overview of the Synthesis of Nucleoside UNIT 13.1 Phosphates and Polyphosphates Phosphorylated nucleosides play a domi- ity to the synthesis. Side reactions can occur, nant role in biochemistry. Primary metabolism, such as depurination of the nucleoside, phos- DNA replication and repair, RNA synthesis, phorylation of the nucleobase, as well as chemi- protein synthesis, signal transduction, polysac- cal alteration of nucleobase analogs. Due to charide biosynthesis, and enzyme regulation their intrinsic reactivity, the synthesis of phos- are just a handful of processes involving these phoanhydride bonds is also synthetically chal- molecules. Literally thousands of enzymes use lenging. Phosphate anhydrides are phosphory- these compounds as substrates and/or regula- lating reagents that are readily degraded under tors. The need to obtain such compounds in acidic conditions. Finally, purification of syn- both labeled and unlabeled forms, as well as a thetic nucleotides can be problematic. Ionic burgeoning need for analogs, has driven the reagents, starting materials, and mixtures of development of a myriad of chemical and en- regioisomers (2′-, 3′-, 5′-phosphates) can be zymatic synthetic approaches. As chemical en- particularly difficult to separate from the de- tities, few molecules possess the wide array of sired product. densely packed functionality present in phos- In spite of the many potential difficulties phorylated nucleosides. This poses a formida- associated with nucleoside phosphorylation ble challenge to the synthetic chemist, one that and polyphosphorylation, a certain amount of has not yet been fully overcome. This overview success has been achieved in these areas. Given will address some common methods (synthetic the wealth of phosphorylating reagents avail- and enzymatic) used to construct phosphory- able, simple phosphorylation of nucleosides at lated nucleosides.