Genetic and Environmental Influences on Human Behavioral Differences
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Effect of Reproductive Compensation on Recessive Disorders Within Consanguineous Human Populations
Heredity (2002) 88, 474–479 2002 Nature Publishing Group All rights reserved 0018-067X/02 $25.00 www.nature.com/hdy The effect of reproductive compensation on recessive disorders within consanguineous human populations ADJ Overall1,2, M Ahmad1 and RA Nichols1 1School of Biological Sciences, Queen Mary, University of London, UK We investigate the effects of consanguinity and population results show how reproductive compensation can effectively substructure on genetic health using the UK Asian popu- counteract the purging of deleterious alleles within con- lation as an example. We review and expand upon previous sanguineous populations. Whereas inbreeding does not treatments dealing with the deleterious effects of con- elevate the equilibrium frequency of affected individuals, sanguinity on recessive disorders and consider how other reproductive compensation does. We suggest this effect factors, such as population substructure, may be of equal must be built into interpretations of the incidence of genetic importance. For illustration, we quantify the relative risks of disease within populations such as the UK Asians. Infor- recessive lethal disorders by presenting some simple calcu- mation of this nature will benefit health care workers who lations that demonstrate the effect ‘reproductive compen- inform such communities. sation’ has on the maintenance of recessive alleles. The Heredity (2002) 88, 474–479. DOI: 10.1038/sj/hdy/6800090 Keywords: marriage; inbreeding; mortality; morbidity; genetic counselling Introduction whether reproductive compensation, at the level ident- ified by Koeslag and Schach (1984), can effectively The majority of the UK Asian population can trace their counteract the purging of deleterious alleles within con- origins to the Punjab region of India and Pakistan within sanguineous populations. -

(1908): Recent Studies in Human Heredity
NOTES AND LITERATURE HEREDITY Recent Studies in Human Heredity.-j\Iustthe fallacy always persist that all ancient and powerful families are necessarily degenerate? As long ago as 1881, Paul Jacoby wrote a book1 to prove that the assumptionof rank and power has always been followedby mentaland physicaldeteriorations ending in sterility and the extinctionof the race. By collectingtogether all evi- dence supportinghis preconceivedtheory, by tracing only the well-knownfamilies in whichpathological conditions were heredi- tary,by failing to treat of dozens of otherswhose recordswould not have supportedhis thesis,by saying everythinghe possibly could that was bad about everyone (followingalways the hostile historians), by ignoring everywherethe normal and virtuous members,he was able to presentwhat was to the uninformedan apparently overwhelmingarray of proof. In regard to the injustice of this one-sided picture I have already had some- thingto say in "M'Nlentaland M\IoralHeredity in Royalty, first publishedsome six years ago. A furtherstudy based upon Jacoby's unsound foundations has recentlycome to my notice,3and althougha well-miadebook containingan interestingseries of 278 portrait illustrations,is necessarilyquite as misleadingas the older structureon which it rests. The main idea of Dr. Galippe is to show that the great swollen protrudingunderlip which descended amionogthe Hiaps- burgs of Austria, Spain and allied houses,and also the protrud- ing underjaw (progil)athismine in e'rieuitr), are stigmata of de- generacy,and to demonstratethis he places beside his portraits, quotationsfrom the writingsof Jacoby. Galippe uses no statistical methods, not even arithmetical counting,and appears to be totally ignorant of English bio- metric writings. His general conclusionsabout the causes of degeneracy (aristocratic environment,etc.) are quite as mis- Etudes sur la selection chez I homem. -

AIH Chapter 2: Human Behavior
Aviation Instructor's Handbook (FAA-H-8083-9) Chapter 2: Human Behavior Introduction Derek’s learner, Jason, is very smart and able to retain a lot of information, but has a tendency to rush through the less exciting material and shows interest and attentiveness only when performing tasks that he finds to be interesting. This concerns Derek because he is worried that Jason will overlook many important details and rush through procedures. For a homework assignment Jason was told to take a very thorough look at Preflight Procedures and that for his next flight lesson they would discuss each step in detail. As Derek predicted, Jason found this assignment to be boring and was not prepared. Derek knows that Jason is a “thrill seeker” as he talks about his business, which is a wilderness adventure company. Derek wants to find a way to keep Jason focused and help him find excitement in all areas of learning so that he will understand the complex art of flying and aircraft safety. Learning is the acquisition of knowledge or understanding of a subject or skill through education, experience, practice, or study. This chapter discusses behavior and how it affects the learning process. An instructor seeks to understand why people act the way they do and how people learn. An effective instructor uses knowledge of human behavior, basic human needs, the defense mechanisms humans use that prevent learning, and how adults learn in order to organize and conduct productive learning activities. Definitions of Human Behavior The study of human behavior is an attempt to explain how and why humans function the way they do. -

The Genetics of Tobacco Use: Methods, Findings and Policy Implications W Hall, P Madden, M Lynskey
119 Tob Control: first published as 10.1136/tc.11.2.119 on 1 June 2002. Downloaded from REVIEW The genetics of tobacco use: methods, findings and policy implications W Hall, P Madden, M Lynskey ............................................................................................................................. Tobacco Control 2002;11:119–124 Research on the genetics of smoking has increased our Specific study designs—adoption, twin, and link- understanding of nicotine dependence, and it is likely to age designs—are needed to separate the effects of genes from those of the environment. illuminate the mechanisms by which cigarette smoking adversely effects the health of smokers. Given recent Adoption studies advances in molecular biology, including the completion In the adoption design we compare the similarity in smoking between adoptive children and their of the draft sequence of the human genome, interest has biological parents with the similarity between now turned to identifying gene markers that predict a adopted children and their adoptive parents or heightened risk of using tobacco and developing between adoptive sibling pairs and biological sib- ling pairs. If smoking is largely or wholly geneti- nicotine dependence cally determined, then there should be greater .......................................................................... similarity between children and their biological parents and siblings than between adopted hanks to RA Fisher, genetics has been linked children and their adoptive parents or siblings. to scepticism about a causal relation between One early adoption study found an association cigarette smoking and lung cancer.12 Fisher3 between the smoking of foster children and their T biological siblings but not their adoptive argued that the association between smoking and 5 6 lung cancer was explained by shared genes that siblings. -

Nurture Might Be Nature: Cautionary Tales and Proposed Solutions Sara
Nurture might be nature: Cautionary tales and proposed solutions Sara A. Hart* Department of Psychology and Florida Center for Reading Research Florida State University, USA Callie Little Department of Psychology University of New England, Australia Florida Center for Reading Research Florida State University, USA Elsje van Bergen Department of Biological Psychology Vrije Universiteit Amsterdam, the Netherlands *Corresponding author: Sara A. Hart, 1107 W. Call Street, Tallahassee, FL, 32308. [email protected], ORCID: 0000-0001-9793-0420 FINAL IN PRESS CITATION: Hart, S.A., Little, C., & van Bergen, E. (in press). Nurture might be nature: Cautionary tales and proposed solutions. npj Science of Learning. 1 Abstract Across a wide range of studies, researchers often conclude that the home environment and children’s outcomes are causally linked. In contrast, behavioral genetic studies show that parents influence their children by providing them with both environment and genes, meaning the environment that parents provide should not be considered in the absence of genetic influences, because that can lead to erroneous conclusions on causation. This article seeks to provide behavioral scientists with a synopsis of numerous methods to estimate the direct effect of the environment, controlling for the potential of genetic confounding. Ideally, using genetically- sensitive designs can fully disentangle this genetic confound, but these require specialized samples. In the near future, researchers will likely have access to measured DNA variants (summarized in a polygenic scores), which could serve as a partial genetic control, but that is currently not an option that is ideal or widely available. We also propose a work around for when genetically sensitive data are not readily available: the Familial Control Method. -

Inheriting Genetic Conditions
Help Me Understand Genetics Inheriting Genetic Conditions Reprinted from MedlinePlus Genetics U.S. National Library of Medicine National Institutes of Health Department of Health & Human Services Table of Contents 1 What does it mean if a disorder seems to run in my family? 1 2 Why is it important to know my family health history? 4 3 What are the different ways a genetic condition can be inherited? 6 4 If a genetic disorder runs in my family, what are the chances that my children will have the condition? 15 5 What are reduced penetrance and variable expressivity? 18 6 What do geneticists mean by anticipation? 19 7 What are genomic imprinting and uniparental disomy? 20 8 Are chromosomal disorders inherited? 22 9 Why are some genetic conditions more common in particular ethnic groups? 23 10 What is heritability? 24 Reprinted from MedlinePlus Genetics (https://medlineplus.gov/genetics/) i Inheriting Genetic Conditions 1 What does it mean if a disorder seems to run in my family? A particular disorder might be described as “running in a family” if more than one person in the family has the condition. Some disorders that affect multiple family members are caused by gene variants (also known as mutations), which can be inherited (passed down from parent to child). Other conditions that appear to run in families are not causedby variants in single genes. Instead, environmental factors such as dietary habits, pollutants, or a combination of genetic and environmental factors are responsible for these disorders. It is not always easy to determine whether a condition in a family is inherited. -

The Psychology of Music Haverford College Psychology 303
The Psychology of Music Haverford College Psychology 303 Instructor: Marilyn Boltz Office: Sharpless 407 Contact Info: 610-896-1235 or [email protected] Office Hours: before class and by appointment Course Description Music is a human universal that has been found throughout history and across different cultures of the world. Why, then, is music so ubiquitous and what functions does it serve? The intent of this course is to examine this question from multiple psychological perspectives. Within a biological framework, it is useful to consider the evolutionary origins of music, its neural substrates, and the development of music processing. The field of cognitive psychology raises questions concerning the relationship between music and language, and music’s ability to communicate emotive meaning that may influence visual processing and body movement. From the perspectives of social and personality psychology, music can be argued to serve a number of social functions that, on a more individual level, contribute to a sense of self and identity. Lastly, musical behavior will be considered in a number of applied contexts that include consumer behavior, music therapy, and the medical environment. Prerequisites: Psychology 100, 200, and at least one advanced 200-level course. Biological Perspectives A. Evolutionary Origins of Music When did music evolve in the overall evolutionary scheme of events and why? Does music serve any adaptive purposes or is it, as some have argued, merely “auditory cheesecake”? What types of evidence allows us to make inferences about the origins of music? Reading: Thompson, W.F. (2009). Origins of Music. In W.F. Thompson, Music, thought, and feeling: Understanding the psychology of music. -

Ancient Genomes Provide Insights Into Family Structure and the Heredity of Social Status in the Early Bronze Age of Southeastern Europe A
www.nature.com/scientificreports OPEN Ancient genomes provide insights into family structure and the heredity of social status in the early Bronze Age of southeastern Europe A. Žegarac1,2, L. Winkelbach2, J. Blöcher2, Y. Diekmann2, M. Krečković Gavrilović1, M. Porčić1, B. Stojković3, L. Milašinović4, M. Schreiber5, D. Wegmann6,7, K. R. Veeramah8, S. Stefanović1,9 & J. Burger2* Twenty-four palaeogenomes from Mokrin, a major Early Bronze Age necropolis in southeastern Europe, were sequenced to analyse kinship between individuals and to better understand prehistoric social organization. 15 investigated individuals were involved in genetic relationships of varying degrees. The Mokrin sample resembles a genetically unstructured population, suggesting that the community’s social hierarchies were not accompanied by strict marriage barriers. We fnd evidence for female exogamy but no indications for strict patrilocality. Individual status diferences at Mokrin, as indicated by grave goods, support the inference that females could inherit status, but could not transmit status to all their sons. We further show that sons had the possibility to acquire status during their lifetimes, but not necessarily to inherit it. Taken together, these fndings suggest that Southeastern Europe in the Early Bronze Age had a signifcantly diferent family and social structure than Late Neolithic and Early Bronze Age societies of Central Europe. Kinship studies in the reconstruction of prehistoric social structure. An understanding of the social organization of past societies is crucial to understanding recent human evolution, and several generations of archaeologists and anthropologists have worked to develop a suite of methods, both scientifc and conceptual, for detecting social conditions in the archaeological record 1–4. -

Social Psychology Glossary
Social Psychology Glossary This glossary defines many of the key terms used in class lectures and assigned readings. A Altruism—A motive to increase another's welfare without conscious regard for one's own self-interest. Availability Heuristic—A cognitive rule, or mental shortcut, in which we judge how likely something is by how easy it is to think of cases. Attractiveness—Having qualities that appeal to an audience. An appealing communicator (often someone similar to the audience) is most persuasive on matters of subjective preference. Attribution Theory—A theory about how people explain the causes of behavior—for example, by attributing it either to "internal" dispositions (e.g., enduring traits, motives, values, and attitudes) or to "external" situations. Automatic Processing—"Implicit" thinking that tends to be effortless, habitual, and done without awareness. B Behavioral Confirmation—A type of self-fulfilling prophecy in which people's social expectations lead them to behave in ways that cause others to confirm their expectations. Belief Perseverance—Persistence of a belief even when the original basis for it has been discredited. Bystander Effect—The tendency for people to be less likely to help someone in need when other people are present than when they are the only person there. Also known as bystander inhibition. C Catharsis—Emotional release. The catharsis theory of aggression is that people's aggressive drive is reduced when they "release" aggressive energy, either by acting aggressively or by fantasizing about aggression. Central Route to Persuasion—Occurs when people are convinced on the basis of facts, statistics, logic, and other types of evidence that support a particular position. -

Influences on Your Health
InfluencesInfluences onon YourYour HealthHealth ChapterChapter 11 LessonLesson 22 HeredityHeredity • Heredity: all the traits and properties that are passed along biologically from both parents to child. • To some degree this determines your general level of health. • You inherit physical traits such as the color of your hair and eyes, shape of your nose and ears, as well as your body type and size. • You also inherit basic intellectual abilities as well as tendencies toward specific diseases. EnvironmentEnvironment • Environment: The sum of your total surroundings- your family, where you grew up, where you live now, and all of your experiences. • Your environment also includes the people in your life as well as your culture. PhysicalPhysical EnvironmentEnvironment • Your physical environment can affect all areas of your health. • Positive environmental influences include: parks, jogging paths, recreational facilities, health care facilities, low crime. • Negative environmental influences include: pollutants such as smog and smoke, high crime, poor access to medical care, exposure to diseases. SocialSocial EnvironmentEnvironment • Your social environment includes your family and other people you come into contact daily. A person who is surrounded by individuals who show love, support, strength, and encouragement is said to have a positive social environment. A healthful positive social environment can help a person rise above adverse physical conditions and create a positive self-image. • A person from an unhealthful social environment may suffer from poor mental and emotional health as a result of rejection, neglect, verbal abuse, and other negative behaviors. SocialSocial EnvironmentEnvironment cont.cont. • An important part of your social environment, especially during the teen years is your peers. -

Nature Vs. Nurture Results in a Draw, According to Twins Meta-Study 19 May 2015
Nature vs. nurture results in a draw, according to twins meta-study 19 May 2015 said. Although the contribution of genetic and environmental factors was balanced for most of the traits studied, the research showed there could be significant differences in individual traits. For example, risk for bipolar disorder was about 70 per cent due to genetics and 30 per cent due to environmental factors. "When visiting the nature versus nurture debate, there is overwhelming evidence that both genetic and environmental factors can influence traits and The research drew on data from almost every twin study diseases," Dr Benyamin said. across the world from the past 50 years. "What is comforting is that, on average, about 50 per cent of individual differences are genetic and 50 per cent are environmental. One of the great tussles of science – whether our health is governed by nature or nurture – has been "The findings show that we need to look at settled, and it is effectively a draw. ourselves outside of a view of nature versus nurture , and instead look at it as nature and nurture." University of Queensland researcher Dr Beben Benyamin from the Queensland Brain Institute In 69 per cent of cases, the study also revealed that collaborated with researchers at VU University of individual traits were the product of the cumulative Amsterdam to review almost every twin study effect of genetic differences. across the world from the past 50 years, involving more than 14.5 million twin pairs. "This means that there are good reasons to study the biology of human traits, and that the combined The findings, published in Nature Genetics, reveal effect of many genes on a trait is simply the sum of on average the variation for human traits and the effect of each individual gene," Dr Benyamin diseases is 49 per cent genetic, and 51 per cent said. -
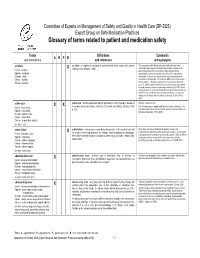
Glossary of Terms Related to Patient and Medication Safety
Committee of Experts on Management of Safety and Quality in Health Care (SP-SQS) Expert Group on Safe Medication Practices Glossary of terms related to patient and medication safety Terms Definitions Comments A R P B and translations and references and synonyms accident accident : an unplanned, unexpected, and undesired event, usually with adverse “For many years safety officials and public health authorities have Xconsequences (Senders, 1994). discouraged use of the word "accident" when it refers to injuries or the French : accident events that produce them. An accident is often understood to be Spanish : accidente unpredictable -a chance occurrence or an "act of God"- and therefore German : Unfall unavoidable. However, most injuries and their precipitating events are Italiano : incidente predictable and preventable. That is why the BMJ has decided to ban the Slovene : nesreča word accident. (…) Purging a common term from our lexicon will not be easy. "Accident" remains entrenched in lay and medical discourse and will no doubt continue to appear in manuscripts submitted to the BMJ. We are asking our editors to be vigilant in detecting and rejecting inappropriate use of the "A" word, and we trust that our readers will keep us on our toes by alerting us to instances when "accidents" slip through.” (Davis & Pless, 2001) active error X X active error : an error associated with the performance of the ‘front-line’ operator of Synonym : sharp-end error French : erreur active a complex system and whose effects are felt almost immediately. (Reason, 1990, This definition has been slightly modified by the Institute of Medicine : “an p.173) error that occurs at the level of the frontline operator and whose effects are Spanish : error activo felt almost immediately.” (Kohn, 2000) German : aktiver Fehler Italiano : errore attivo Slovene : neposredna napaka see also : error active failure active failures : actions or processes during the provision of direct patient care that Since failure is a term not defined in the glossary, its use is not X recommended.