Syntheses and Applications of Trifluoromethyl- and Pentafluorosulfanyl-Containing Organic Molecules Siyan Qing Clemson University, [email protected]
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
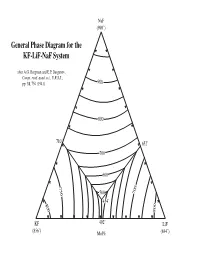
General Phase Diagram for the KF-Lif-Naf System
NaF (990˚) General Phase Diagram for the KF-LiF-NaF System after A.G. Bergman and E.P. Dergunov, Compt. rend. acad. sci., U.R.S.S., pp. 31, 754 (1941). 900 800 710˚ 652˚ 700 600 750 500 700 454˚ 800 800 KF 492˚ LiF (856˚) Mol% (844˚) Sodium Fluoride NaF 950˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 940˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 930˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 920˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 910˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 900˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 890˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 880˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 870˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 860˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 850˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 840˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 830˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 820˚ KF LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 810˚ 810˚ ˚ KF 810 LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 800˚ 800˚ ˚ KF 800 LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 790˚ 790˚ ˚ KF 790 LiF Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 780˚ 780˚ ˚ LiF KF 780 Potassium Fluoride Lithium Fluoride Sodium Fluoride NaF 770˚ 770˚ ˚ LiF KF 770 Potassium Fluoride -

Acidity, Basicity, and Pka 8 Connections
Acidity, basicity, and pKa 8 Connections Building on: Arriving at: Looking forward to: • Conjugation and molecular stability • Why some molecules are acidic and • Acid and base catalysis in carbonyl ch7 others basic reactions ch12 & ch14 • Curly arrows represent delocalization • Why some acids are strong and others • The role of catalysts in organic and mechanisms ch5 weak mechanisms ch13 • How orbitals overlap to form • Why some bases are strong and others • Making reactions selective using conjugated systems ch4 weak acids and bases ch24 • Estimating acidity and basicity using pH and pKa • Structure and equilibria in proton- transfer reactions • Which protons in more complex molecules are more acidic • Which lone pairs in more complex molecules are more basic • Quantitative acid/base ideas affecting reactions and solubility • Effects of quantitative acid/base ideas on medicine design Note from the authors to all readers This chapter contains physical data and mathematical material that some readers may find daunting. Organic chemistry students come from many different backgrounds since organic chemistry occu- pies a middle ground between the physical and the biological sciences. We hope that those from a more physical background will enjoy the material as it is. If you are one of those, you should work your way through the entire chapter. If you come from a more biological background, especially if you have done little maths at school, you may lose the essence of the chapter in a struggle to under- stand the equations. We have therefore picked out the more mathematical parts in boxes and you should abandon these parts if you find them too alien. -

Trifluoromethane)
SAFETY DATA SHEET Halocarbon R-23 (Trifluoromethane) Section 1. Identification GHS product identifier : Halocarbon R-23 (Trifluoromethane) Chemical name : trifluoromethane Other means of : Fluoroform; Arcton 1; Fluoryl; Freon F-23; Freon 23; Genetron 23; Methyl trifluoride; R identification 23; Trifluoromethane; CHF3; Arcton; Halocarbon 23; UN 1984; Carbon trifluoride; Genetron HFC23; Propellant 23; Refrigerant 23 Product type : Liquefied gas Product use : Synthetic/Analytical chemistry. Synonym : Fluoroform; Arcton 1; Fluoryl; Freon F-23; Freon 23; Genetron 23; Methyl trifluoride; R 23; Trifluoromethane; CHF3; Arcton; Halocarbon 23; UN 1984; Carbon trifluoride; Genetron HFC23; Propellant 23; Refrigerant 23 SDS # : 001078 Supplier's details : Airgas USA, LLC and its affiliates 259 North Radnor-Chester Road Suite 100 Radnor, PA 19087-5283 1-610-687-5253 24-hour telephone : 1-866-734-3438 Section 2. Hazards identification OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the : GASES UNDER PRESSURE - Liquefied gas substance or mixture GHS label elements Hazard pictograms : Signal word : Warning Hazard statements : Contains gas under pressure; may explode if heated. May cause frostbite. May displace oxygen and cause rapid suffocation. Precautionary statements General : Read and follow all Safety Data Sheets (SDS’S) before use. Read label before use. Keep out of reach of children. If medical advice is needed, have product container or label at hand. Close valve after each use and when empty. Use equipment rated for cylinder pressure. Do not open valve until connected to equipment prepared for use. Use a back flow preventative device in the piping. Use only equipment of compatible materials of construction. -

Classification of Medicinal Drugs and Driving: Co-Ordination and Synthesis Report
Project No. TREN-05-FP6TR-S07.61320-518404-DRUID DRUID Driving under the Influence of Drugs, Alcohol and Medicines Integrated Project 1.6. Sustainable Development, Global Change and Ecosystem 1.6.2: Sustainable Surface Transport 6th Framework Programme Deliverable 4.4.1 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Due date of deliverable: 21.07.2011 Actual submission date: 21.07.2011 Revision date: 21.07.2011 Start date of project: 15.10.2006 Duration: 48 months Organisation name of lead contractor for this deliverable: UVA Revision 0.0 Project co-funded by the European Commission within the Sixth Framework Programme (2002-2006) Dissemination Level PU Public PP Restricted to other programme participants (including the Commission x Services) RE Restricted to a group specified by the consortium (including the Commission Services) CO Confidential, only for members of the consortium (including the Commission Services) DRUID 6th Framework Programme Deliverable D.4.4.1 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Page 1 of 243 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Authors Trinidad Gómez-Talegón, Inmaculada Fierro, M. Carmen Del Río, F. Javier Álvarez (UVa, University of Valladolid, Spain) Partners - Silvia Ravera, Susana Monteiro, Han de Gier (RUGPha, University of Groningen, the Netherlands) - Gertrude Van der Linden, Sara-Ann Legrand, Kristof Pil, Alain Verstraete (UGent, Ghent University, Belgium) - Michel Mallaret, Charles Mercier-Guyon, Isabelle Mercier-Guyon (UGren, University of Grenoble, Centre Regional de Pharmacovigilance, France) - Katerina Touliou (CERT-HIT, Centre for Research and Technology Hellas, Greece) - Michael Hei βing (BASt, Bundesanstalt für Straßenwesen, Germany). -

Survey and Risk Assessment of Chemical Substances in Deodorants
Survey and risk assessment of chemical substances in deodorants Suresh C. Rastogi & Gitte Hellerup Jensen National Environmental Research Institute Jeanne Duus Johansen National Allergy Research Centre Survey of Chemical Substances in Consumer Products, No. 86 2007 The Danish Environmental Protection Agency will, when opportunity offers, publish reports and contributions relating to environmental research and development projects financed via the Danish EPA. Please note that publication does not signify that the contents of the reports necessarily reflect the views of the Danish EPA. The reports are, however, published because the Danish EPA finds that the studies represent a valuable contribution to the debate on environmental policy in Denmark. Contents SAMMENFATNING 5 SUMMARY 7 1 INTRODUCTION 11 2 MARKET SURVEY AND PRODUCT SAMPLING 13 2.1 MARKET SURVEY 13 2.2 LEGISLATION 15 2.3 SAMPLING OF PRODUCTS AND CONTROL OF LABELLING 15 2.4 SELECTION OF PRODUCTS FOR ANALYSIS 18 3 ANALYSIS 19 3.1 MATERIALS 19 3.2 ANALYSIS 19 3.2.1 Sample preparation 19 3.2.2 Analysis of fragrance substances 19 3.2.3 Analysis of triclosan 20 4 RESULTS 21 5 RISK ASSESSMENT 27 5.1 DEODORANTS AND CONTACT ALLERGY 27 5.2 RISK ASSESSMENT – IN GENERAL 27 5.3 THE SELECTED FRAGRANCE SUBSTANCES 28 5.3.1 HYDOXYISOHEXYL 3-CYCLOHEXENE CARBOXALDEHYDE (HICC) 28 5.3.2 HYDROXYCITRONELLAL 32 5.3.3 ISOEUGENOL 33 5.3.4 CINNAMAL/CINNAMYL ALCOHOL 34 5.4 FARNESOL 35 5.5 COMMENTS CONCERNING OTHER FRAGRANCE SUBSTANCES 35 5.6 ALLERGEN LOAD OF FRAGRANCE SUBSTANCES 36 5.7 TRICLOSAN 36 6 DISCUSSION 38 7 REFERENCES 43 ANNEX 1 49 3 4 Sammenfatning Deodoranter anvendes dagligt af store dele af befolkningen og kan indeholde ingredienser, som visse duftstoffer og konserveringsmidler, der er hyppige år- sager til hudallergi. -

From Celebrex to a Novel Class of Phosphoinositide-Dependent Kinase 1 (Pdk-1) Inhibitors for Androgen-Independent Prostate Caner
FROM CELEBREX TO A NOVEL CLASS OF PHOSPHOINOSITIDE-DEPENDENT KINASE 1 (PDK-1) INHIBITORS FOR ANDROGEN-INDEPENDENT PROSTATE CANER DISSERTATION Presented in Partial Fulfillment of the requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Jiuxiang Zhu, M.S. * * * * * The Ohio State University 2005 Dissertation Committee: Approved by Professor Ching-Shih Chen, Advisor Professor Robert Brueggemeier Advisor Professor Pui-Kai (Tom) Li Graduate Program in Pharmacy Professor Matthew D. Ringel ABSTRACT Celebrex, a nonsteroidal anti-inflammatory drug (NSAID, cyclooxygenase-2 inhibitor), was reported to induce apoptosis in the prostate cancer cell line PC-3 at 50µM. Early research from our laboratory demonstrated that this apoptotic inducing effect was independent of its COX-2 inhibitory activity. Further investigation showed that PDK-1 was a major protein targeted by celebrex in PC-3 cells to induce apoptosis. However, celebrex was very weak in inhibiting PDK-1 with IC50 of 48µM. To improve its potency, two series of analogs were designed and synthesized. In the first series of 24 compounds, the 5-position methylphenyl moiety of celebrex was replaced by various aromatic ring systems to explore the optimal hydrophobic group. OSU02067 (IC50=9µM) with phenanthrene at the 5-position was the best inhibitor among this series and was selected as the lead compound for further modification. Enzyme kinetics study of PDK-1 inhibition by celebrex indicated that it competed with ATP for binding. Docking of OSU02067 into the ATP binding domain of PDK-1 showed that the sulfonamide moiety hydrogen bonded to hinge region residue Ala162. -

Refrigerant Safety Refrigerant History
Refrigerant Safety The risks associated with the use of refrigerants in refrigeration and airconditioning equipment can include toxicity, flammability, asphyxiation, and physical hazards. Although refrigerants can pose one or more of these risks, system design, engineering controls, and other techniques mitigate this risk for the use of refrigerant in various types of equipment. Refrigerant History Nearly all of the historically used refrigerants were flammable, toxic, or both. Some were also highly reactive, resulting in accidents (e. g., leak, explosion) due to equipment failure, poor maintenance, or human error. The task of finding a nonflammable refrigerant with good stability was given to Thomas Midgley in 1926. With his associates Albert Leon Henne and Robert Reed McNary, Dr. Midgley observed that the refrigerants then in use comprised relatively few chemical elements, many of which were clustered in an intersecting row and column of the periodic table of elements. The element at the intersection was fluorine, known to be toxic by itself. Midgley and his collaborators felt, however, that compounds containing fluorine could be both nontoxic and nonflammable. The attention of Midgley and his associates was drawn to organic fluorides by an error in the literature that showed the boiling point for tetrafluoromethane (carbon tetrafluoride) to be high compared to those for other fluorinated compounds. The correct boiling temperature later was found to be much lower. Nevertheless, the incorrect value was in the range sought and led to evaluation of organic fluorides as candidates. The shorthand convention, later introduced to simplify identification of the organic fluorides for a systematic search, is used today as the numbering system for refrigerants. -

Status of Industry Efforts to Replace Halon Fire Extinguishing Agents
STATUS OF INDUSTRY EFFORTS TO REPLACE HALON FIRE EXTINGUISHING AGENTS Robert T. Wickham, P.E. March 16, 2002 WICKHAM ASSOCIATES NOTICE This document is disseminated under the sponsorship of the U.S. Environmental Protection Agency in the interest of information exchange. The views expressed in this report are those of the author and do not necessarily reflect those of the US Environmental Protection Agency. The US Environmental Protection Agency does not assume liability for the contents or use thereof and further the Agency does not endorse products or manufacturers. Trade or manufacturer's names appear herein solely because they are considered essential to the objective of this report. The following commercial products (requiring a trademark designation ® or ™) are mentioned in this report. Due to the frequency of usage, trademarks are not indicated. Mention of a product does not constitute endorsement or rejection of the product. Ansul FE-25 Argonite FE-36 Argotec FM-200 CEA-410 Halotron I CEA-614 Inergen CEA-308 NAF P-IV Envirogel NAF S-III FE-13 NN100 FE-227 Triodide FE-241 There are no restrictions on copying or distribution of this document. Additional copies of this report are available from ….. Wickham Associates 9 Winding Brook Drive Stratham, New Hampshire 03885 USA Tel: +603-772-3229 Fax: +603-772-7305 email: [email protected] The report is available at http://home.attbi.com/~wickham/downloads.htm for downloading in Adobe Acrobat portable document format (pdf). Preface This report is not intended to be a market study, but instead a snapshot of the progress industry and the government are making in employing non ozone depleting alternatives to halons in the fire protection sector. -

Shelf-Stable Electrophilic Trifluoromethylating Reagents: a Brief Historical Perspective
Shelf-stable electrophilic trifluoromethylating reagents: A brief historical perspective Norio Shibata*1, Andrej Matsnev1 and Dominique Cahard*2 Review Open Access Address: Beilstein J. Org. Chem. 2010, 6, No. 65. doi:10.3762/bjoc.6.65 1Department of Frontier Materials, Graduate School of Engineering, Nagoya Institute of Technology, Gokiso, Showa-ku, Nagoya Received: 15 March 2010 466-8555, Japan and 2UMR 6014 CNRS – C.O.B.R.A. Université et Accepted: 21 May 2010 INSA de Rouen, 1 rue Tesnière, 76130 Mont Saint Aignan, France Published: 16 June 2010 Email: Guest Editor: D. O'Hagan Norio Shibata* - [email protected]; Dominique Cahard* - [email protected] © 2010 Shibata et al; licensee Beilstein-Institut. License and terms: see end of document. * Corresponding author Keywords: asymmetric synthesis; electrophilic; fluorine; reagent; trifluoromethylation Abstract Since the discovery by Yagupolskii and co-workers that S-trifluoromethyl diarylsulfonium salts are effective for the trifluoro- methylation of thiophenolates, the design and synthesis of electrophilic trifluoromethylating reagents have been extensively researched in both academia and industry, due to the significant unique features that trifluoromethylated compounds have in phar- maceuticals, agricultural chemicals, and functional materials. Several effective reagents have been developed by the groups of Yagupolskii, Umemoto, Shreeve, Adachi, Magnier, Togni and Shibata. Due to the high stability and reactivity of these reagents, a series of Umemoto reagents, Togni reagent and Shibata reagent are now commercially available. In this review, we wish to briefly provide a historical perspective of the development of so-called “shelf-stable electrophilic trifluoromethylating reagents”, although this field is in constant development. Review The chemistry of fluoro-organic compounds is one of the areas [3]. -

Naming Molecular Compounds General Instructions: Please Do the Activities for Each Day As Indicated
Teacher Name: Dwight Lillie Student Name: ________________________ Class: ELL Chemistry Period: Per 4 Assignment: Assignment week 2 Due: Friday, 5/8 Naming Molecular Compounds General Instructions: Please do the activities for each day as indicated. Any additional paper needed please attach. Submitted Work: 1) Completed packet. Questions: Please send email to your instructor and/or attend published virtual office hours. Schedule: Date Activity Monday (4/27) Read Sections 9.3, 9.5 in your textbook. Tuesday (4/28) Read and work through questions 1-9 Wednesday (4/29) Read and work through questions 10-14 Thursday (4/30) Read and work through questions 14-18 Friday (5/31) Read and work through questions 19-21 How are the chemical formula and name of a molecular compound related? Why? When you began chemistry class this year, you probably already knew that the chemical formula for carbon dioxide was CO2. Today you will find out why CO2 is named that way. Naming chemical compounds correctly is of paramount importance. The slight difference between the names carbon monoxide (CO, a poisonous, deadly gas) and carbon dioxide (CO2, a greenhouse gas that we exhale when we breathe out) can be the difference between life and death! In this activity you will learn the naming system for molecular compounds. Model 1 – Molecular Compounds Molecular Number of Atoms Number of Atoms in Name of Compound Formula of First Element Second Element ClF Chlorine monofluoride ClF5 1 5 Chlorine pentafluoride CO Carbon monoxide CO2 Carbon dioxide Cl2O Dichlorine monoxide PCl5 Phosphorus pentachloride N2O5 Dinitrogen pentoxide 1. Fill in the table to indicate the number of atoms of each type in the molecular formula. -

Liquefied Gas Conversion Chart
LIQUEFIED GAS CONVERSION CHART Cubic Feet / Pound Pounds / Gallon Product Name Column A Column B Acetylene UN/NA: 1001 14.70 4.90 CAS: 514-86-2 Air UN/NA: 1002 13.30 7.29 CAS: N/A Ammonia Anhydrous UN/NA: 1005 20.78 5.147 CAS: 7664-41-7 Argon UN/NA: 1006 9.71 11.63 CAS: 7440-37-1 Butane UN/NA: 1075 6.34 4.86 CAS: 106-97-8 Carbon Dioxide UN/NA: 2187 8.74 8.46 CAS: 124-38-9 Chlorine UN/NA: 1017 5.38 11.73 CAS: 7782-50-5 Ethane UN/NA: 1045 12.51 2.74 CAS: 74-84-0 Ethylene Oxide UN/NA: 1040 8.78 7.25 CAS: 75-21-8 Fluorine UN/NA: 1045 10.17 12.60 CAS: 7782-41-4 Helium UN/NA: 1046 97.09 1.043 CAS: 7440-59-7 Hydrogen UN/NA: 1049 192.00 0.592 CAS: 1333-74-0 1. Find the gas you want to convert. 2. If you know your quantity in cubic feet and want to convert to pounds, divide your amount by column A 3. If you know your quantity in gallons and want to convert to pounds, multiply your amount by column B 4. If you know your quantity in pounds and want to convert to gallons, divide your amount by column B If you have any questions, please call 1-800-433-2288 LIQUEFIED GAS CONVERSION CHART Cubic Feet / Pound Pounds / Gallon Product Name Column A Column B Hydrogen Chloride UN/NA: 1050 10.60 8.35 CAS: 7647-01-0 Krypton UN/NA: 1056 4.60 20.15 CAS: 7439-90-9 Methane UN/NA: 1971 23.61 3.55 CAS: 74-82-8 Methyl Bromide UN/NA: 1062 4.03 5.37 CAS: 74-83-9 Neon UN/NA: 1065 19.18 10.07 CAS: 7440-01-9 Mapp Gas UN/NA: 1060 9.20 4.80 CAS: N/A Nitrogen UN/NA: 1066 13.89 6.75 CAS: 7727-37-9 Nitrous Oxide UN/NA: 1070 8.73 6.45 CAS: 10024-97-2 Oxygen UN/NA: 1072 12.05 9.52 CAS: 7782-44-7 Propane UN/NA: 1075 8.45 4.22 CAS: 74-98-6 Sulfur Dioxide UN/NA: 1079 5.94 12.0 CAS: 7446-09-5 Xenon UN/NA: 2036 2.93 25.51 CAS: 7440-63-3 1. -

Operation Permit Application
Un; iy^\ tea 0 9 o Operation Permit Application Located at: 2002 North Orient Road Tampa, Florida 33619 (813) 623-5302 o Training Program TRAINING PROGRAM for Universal Waste & Transit Orient Road Tampa, Florida m ^^^^ HAZARDOUS WAb 1 P.ER^AlTTlNG TRAINING PROGRAM MASTER INDEX CHAPTER 1: Introduction Tab A CHAPTER 2: General Safety Manual Tab B CHAPTER 3: Protective Clothing Guide Tab C CHAPTER 4: Respiratory Training Program Tab D APPENDIX 1: Respiratory Training Program II Tab E CHAPTER 5: Basic Emergency Training Guide Tab F CHAPTER 6: Facility Operations Manual Tab G CHAPTER 7: Land Ban Certificates Tab H CHAPTER 8: Employee Certification Statement Tab. I CHAPTER ONE INTRODUCTION prepared by Universal Waste & Transit Orient Road Tampa Florida Introducti on STORAGE/TREATMENT PERSONNEL TRAINING PROGRAM All personnel involved in any handling, transportation, storage or treatment of hazardous wastes are required to start the enclosed training program within one-week after the initiation of employment at Universal Waste & Transit. This training program includes the following: Safety Equipment Personnel Protective Equipment First Aid & CPR Waste Handling Procedures Release Prevention & Response Decontamination Procedures Facility Operations Facility Maintenance Transportation Requirements Recordkeeping We highly recommend that all personnel involved in the handling, transportation, storage or treatment of hazardous wastes actively pursue additional technical courses at either the University of South Florida, or Tampa Junior College. Recommended courses would include general chemistry; analytical chemistry; environmental chemistry; toxicology; and additional safety and health related topics. Universal Waste & Transit will pay all registration, tuition and book fees for any courses which are job related. The only requirement is the successful completion of that course.