Functional Analysis of Ectodysplasin-A Mutations in X-Linked Non-Syndromic Hypodontia and Possible Involvement of X-Chromosome Inactivation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

RT² Profiler PCR Array (384-Well Format) Human Apoptosis 384HT
RT² Profiler PCR Array (384-Well Format) Human Apoptosis 384HT Cat. no. 330231 PAHS-3012ZE For pathway expression analysis Format For use with the following real-time cyclers RT² Profiler PCR Array, Applied Biosystems® models 7900HT (384-well block), Format E ViiA™ 7 (384-well block); Bio-Rad CFX384™ RT² Profiler PCR Array, Roche® LightCycler® 480 (384-well block) Format G Description The Human Apoptosis RT² Profiler PCR Array profiles the expression of 370 key genes involved in apoptosis, or programmed cell death. The array includes the TNF ligands and their receptors; members of the bcl-2 family, BIR (baculoviral IAP repeat) domain proteins, CARD domain (caspase recruitment domain) proteins, death domain proteins, TRAF (TNF receptor-associated factor) domain proteins and caspases. These 370 genes are also grouped by their functional contribution to apoptosis, either anti-apoptosis or induction of apoptosis. Using real-time PCR, you can easily and reliably analyze expression of a focused panel of genes related to apoptosis with this array. For further details, consult the RT² Profiler PCR Array Handbook. Sample & Assay Technologies Shipping and storage RT² Profiler PCR Arrays in formats E and G are shipped at ambient temperature, on dry ice, or blue ice packs depending on destination and accompanying products. For long term storage, keep plates at –20°C. Note: Ensure that you have the correct RT² Profiler PCR Array format for your real-time cycler (see table above). Note: Open the package and store the products appropriately immediately -

Cytokine Nomenclature
RayBiotech, Inc. The protein array pioneer company Cytokine Nomenclature Cytokine Name Official Full Name Genbank Related Names Symbol 4-1BB TNFRSF Tumor necrosis factor NP_001552 CD137, ILA, 4-1BB ligand receptor 9 receptor superfamily .2. member 9 6Ckine CCL21 6-Cysteine Chemokine NM_002989 Small-inducible cytokine A21, Beta chemokine exodus-2, Secondary lymphoid-tissue chemokine, SLC, SCYA21 ACE ACE Angiotensin-converting NP_000780 CD143, DCP, DCP1 enzyme .1. NP_690043 .1. ACE-2 ACE2 Angiotensin-converting NP_068576 ACE-related carboxypeptidase, enzyme 2 .1 Angiotensin-converting enzyme homolog ACTH ACTH Adrenocorticotropic NP_000930 POMC, Pro-opiomelanocortin, hormone .1. Corticotropin-lipotropin, NPP, NP_001030 Melanotropin gamma, Gamma- 333.1 MSH, Potential peptide, Corticotropin, Melanotropin alpha, Alpha-MSH, Corticotropin-like intermediary peptide, CLIP, Lipotropin beta, Beta-LPH, Lipotropin gamma, Gamma-LPH, Melanotropin beta, Beta-MSH, Beta-endorphin, Met-enkephalin ACTHR ACTHR Adrenocorticotropic NP_000520 Melanocortin receptor 2, MC2-R hormone receptor .1 Activin A INHBA Activin A NM_002192 Activin beta-A chain, Erythroid differentiation protein, EDF, INHBA Activin B INHBB Activin B NM_002193 Inhibin beta B chain, Activin beta-B chain Activin C INHBC Activin C NM005538 Inhibin, beta C Activin RIA ACVR1 Activin receptor type-1 NM_001105 Activin receptor type I, ACTR-I, Serine/threonine-protein kinase receptor R1, SKR1, Activin receptor-like kinase 2, ALK-2, TGF-B superfamily receptor type I, TSR-I, ACVRLK2 Activin RIB ACVR1B -

Table 2. Significant
Table 2. Significant (Q < 0.05 and |d | > 0.5) transcripts from the meta-analysis Gene Chr Mb Gene Name Affy ProbeSet cDNA_IDs d HAP/LAP d HAP/LAP d d IS Average d Ztest P values Q-value Symbol ID (study #5) 1 2 STS B2m 2 122 beta-2 microglobulin 1452428_a_at AI848245 1.75334941 4 3.2 4 3.2316485 1.07398E-09 5.69E-08 Man2b1 8 84.4 mannosidase 2, alpha B1 1416340_a_at H4049B01 3.75722111 3.87309653 2.1 1.6 2.84852656 5.32443E-07 1.58E-05 1110032A03Rik 9 50.9 RIKEN cDNA 1110032A03 gene 1417211_a_at H4035E05 4 1.66015788 4 1.7 2.82772795 2.94266E-05 0.000527 NA 9 48.5 --- 1456111_at 3.43701477 1.85785922 4 2 2.8237185 9.97969E-08 3.48E-06 Scn4b 9 45.3 Sodium channel, type IV, beta 1434008_at AI844796 3.79536664 1.63774235 3.3 2.3 2.75319499 1.48057E-08 6.21E-07 polypeptide Gadd45gip1 8 84.1 RIKEN cDNA 2310040G17 gene 1417619_at 4 3.38875643 1.4 2 2.69163229 8.84279E-06 0.0001904 BC056474 15 12.1 Mus musculus cDNA clone 1424117_at H3030A06 3.95752801 2.42838452 1.9 2.2 2.62132809 1.3344E-08 5.66E-07 MGC:67360 IMAGE:6823629, complete cds NA 4 153 guanine nucleotide binding protein, 1454696_at -3.46081884 -4 -1.3 -1.6 -2.6026947 8.58458E-05 0.0012617 beta 1 Gnb1 4 153 guanine nucleotide binding protein, 1417432_a_at H3094D02 -3.13334396 -4 -1.6 -1.7 -2.5946297 1.04542E-05 0.0002202 beta 1 Gadd45gip1 8 84.1 RAD23a homolog (S. -

Mutations in EDA and EDAR Genes in a Large Mexican Hispanic Cohort with Hypohidrotic Ectodermal Dysplasia
Letter to the Editor http://dx.doi.org/10.5021/ad.2015.27.4.474 Mutations in EDA and EDAR Genes in a Large Mexican Hispanic Cohort with Hypohidrotic Ectodermal Dysplasia Julio C Salas-Alanis1,2, Eva Wozniak3, Charles A Mein3, Carola C Duran Mckinster4, Jorge Ocampo-Candiani1, David P Kelsell5, Rong Hua6, Maria L Garza-Rodriguez7, Keith A Choate6, Hugo A Barrera Saldaña7,8 1Dermatology Department, Hospital Universitario, Universidad Autonoma de Nuevo Leon, 2Basic Science Department, Medicine School, Universidad de Monterrey, Monterrey, NL, Mexico, 3Barts and the London Genome Centre, John Vane Science Centre, Barts and the London School of Medicine and Dentistry, University of London, London, United Kingdom, 4Instituto Nacional de Pediatria, Coyoacan, CP, Mexico, 5Centre for Cutaneous Research, Blizard Institute, Barts and the London School of Medicine and Dentistry, Queen Mary University of London, London, United Kingdom, 6Departments of Dermatology, Genetics, and Pathology, Yale University School of Medicine, New Haven, CT, USA, 7Facultad de Medicina, Departamento de Bioquímica y Medicina Molecular, Universidad Autonoma de Nuevo Leon,8Vitagenesis, Monterrey, NL, Mexico Dear Editor: cludes dryness of the skin, eyes, airways, and mucous Ectodermal dysplasias (ED) encompass nearly 200 differ- membranes, as well as other ectodermal defects and, in ent genetic conditions identified by the lack, or dysgenesis, some cases, fever, seizures, and rarely, death. of at least two ectodermal derivatives, such as hair, nails, XL-HED is caused by mutations in the EDA gene, located teeth, and sweat glands. Hypohidrotic/anhidrotic ED (HED) on chromosome Xq12-q13.1, which encodes a signaling is the most frequent form of ED and it can be inherited as molecule of the tumor necrosis factor (TNF) superfamily. -

Functional Analysis of Ectodysplasin-A Mutations in X-Linked Non-Syndromic Hypodontia and Possible Involvement of X-Chromosome Inactivation
Functional analysis of Ectodysplasin-A mutations in X-linked non-syndromic hypodontia and possible involvement of X-chromosome inactivation Yuhua Pan ( [email protected] ) Southern Medical University https://orcid.org/0000-0001-8039-1627 Ting Lu Southern Medical University Ling Peng Southern Medical University Qi Zeng Southern Medical University Xiangyu Huang Southern Medical University Buling Wu Southern Medical University Fu Xiong Southern Medical University Research Article Keywords: ectodysplasin-A, dental pulp stem cells, X chromosome inactivation, tooth agenesis, odontogenic differentiation Posted Date: August 13th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-602940/v2 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/17 Abstract Background: Mutations of the Ectodysplasin-A (EDA) gene are generally associated with syndrome hypohidrotic ectodermal dysplasia or non-syndromic tooth agenesis. The inuence of EDA mutations on dentinogenesis and odontoblast differentiation have not been reported. The aim of this study was to identify genetic clues for the causes of familial non-syndromic oligodontia and explore the underlying mechanisms involved, while focusing on the role of human dental pulp stem cells (hDPSCs). Materials and Methods: Candidate gene sequences were obtained by PCR amplication and Sanger sequencing. Functional analysis was conducted, and the pathogenesis associated with EDA mutations in hDPSCs was investigated to explore the impact of the identied mutation on the phenotype. Capillary electrophoresis (CE) was used to detect X chromosome inactivation (XCI) in the blood of female carriers. Results: In this study, we identied an EDA mutation in a Chinese familythe missense mutation c.1013C>T (Thr338Met). -

Cellular and Molecular Signatures in the Disease Tissue of Early
Cellular and Molecular Signatures in the Disease Tissue of Early Rheumatoid Arthritis Stratify Clinical Response to csDMARD-Therapy and Predict Radiographic Progression Frances Humby1,* Myles Lewis1,* Nandhini Ramamoorthi2, Jason Hackney3, Michael Barnes1, Michele Bombardieri1, Francesca Setiadi2, Stephen Kelly1, Fabiola Bene1, Maria di Cicco1, Sudeh Riahi1, Vidalba Rocher-Ros1, Nora Ng1, Ilias Lazorou1, Rebecca E. Hands1, Desiree van der Heijde4, Robert Landewé5, Annette van der Helm-van Mil4, Alberto Cauli6, Iain B. McInnes7, Christopher D. Buckley8, Ernest Choy9, Peter Taylor10, Michael J. Townsend2 & Costantino Pitzalis1 1Centre for Experimental Medicine and Rheumatology, William Harvey Research Institute, Barts and The London School of Medicine and Dentistry, Queen Mary University of London, Charterhouse Square, London EC1M 6BQ, UK. Departments of 2Biomarker Discovery OMNI, 3Bioinformatics and Computational Biology, Genentech Research and Early Development, South San Francisco, California 94080 USA 4Department of Rheumatology, Leiden University Medical Center, The Netherlands 5Department of Clinical Immunology & Rheumatology, Amsterdam Rheumatology & Immunology Center, Amsterdam, The Netherlands 6Rheumatology Unit, Department of Medical Sciences, Policlinico of the University of Cagliari, Cagliari, Italy 7Institute of Infection, Immunity and Inflammation, University of Glasgow, Glasgow G12 8TA, UK 8Rheumatology Research Group, Institute of Inflammation and Ageing (IIA), University of Birmingham, Birmingham B15 2WB, UK 9Institute of -

Molecular Effects of Isoflavone Supplementation Human Intervention Studies and Quantitative Models for Risk Assessment
Molecular effects of isoflavone supplementation Human intervention studies and quantitative models for risk assessment Vera van der Velpen Thesis committee Promotors Prof. Dr Pieter van ‘t Veer Professor of Nutritional Epidemiology Wageningen University Prof. Dr Evert G. Schouten Emeritus Professor of Epidemiology and Prevention Wageningen University Co-promotors Dr Anouk Geelen Assistant professor, Division of Human Nutrition Wageningen University Dr Lydia A. Afman Assistant professor, Division of Human Nutrition Wageningen University Other members Prof. Dr Jaap Keijer, Wageningen University Dr Hubert P.J.M. Noteborn, Netherlands Food en Consumer Product Safety Authority Prof. Dr Yvonne T. van der Schouw, UMC Utrecht Dr Wendy L. Hall, King’s College London This research was conducted under the auspices of the Graduate School VLAG (Advanced studies in Food Technology, Agrobiotechnology, Nutrition and Health Sciences). Molecular effects of isoflavone supplementation Human intervention studies and quantitative models for risk assessment Vera van der Velpen Thesis submitted in fulfilment of the requirements for the degree of doctor at Wageningen University by the authority of the Rector Magnificus Prof. Dr M.J. Kropff, in the presence of the Thesis Committee appointed by the Academic Board to be defended in public on Friday 20 June 2014 at 13.30 p.m. in the Aula. Vera van der Velpen Molecular effects of isoflavone supplementation: Human intervention studies and quantitative models for risk assessment 154 pages PhD thesis, Wageningen University, Wageningen, NL (2014) With references, with summaries in Dutch and English ISBN: 978-94-6173-952-0 ABSTRact Background: Risk assessment can potentially be improved by closely linked experiments in the disciplines of epidemiology and toxicology. -

Supplementary Table S4. FGA Co-Expressed Gene List in LUAD
Supplementary Table S4. FGA co-expressed gene list in LUAD tumors Symbol R Locus Description FGG 0.919 4q28 fibrinogen gamma chain FGL1 0.635 8p22 fibrinogen-like 1 SLC7A2 0.536 8p22 solute carrier family 7 (cationic amino acid transporter, y+ system), member 2 DUSP4 0.521 8p12-p11 dual specificity phosphatase 4 HAL 0.51 12q22-q24.1histidine ammonia-lyase PDE4D 0.499 5q12 phosphodiesterase 4D, cAMP-specific FURIN 0.497 15q26.1 furin (paired basic amino acid cleaving enzyme) CPS1 0.49 2q35 carbamoyl-phosphate synthase 1, mitochondrial TESC 0.478 12q24.22 tescalcin INHA 0.465 2q35 inhibin, alpha S100P 0.461 4p16 S100 calcium binding protein P VPS37A 0.447 8p22 vacuolar protein sorting 37 homolog A (S. cerevisiae) SLC16A14 0.447 2q36.3 solute carrier family 16, member 14 PPARGC1A 0.443 4p15.1 peroxisome proliferator-activated receptor gamma, coactivator 1 alpha SIK1 0.435 21q22.3 salt-inducible kinase 1 IRS2 0.434 13q34 insulin receptor substrate 2 RND1 0.433 12q12 Rho family GTPase 1 HGD 0.433 3q13.33 homogentisate 1,2-dioxygenase PTP4A1 0.432 6q12 protein tyrosine phosphatase type IVA, member 1 C8orf4 0.428 8p11.2 chromosome 8 open reading frame 4 DDC 0.427 7p12.2 dopa decarboxylase (aromatic L-amino acid decarboxylase) TACC2 0.427 10q26 transforming, acidic coiled-coil containing protein 2 MUC13 0.422 3q21.2 mucin 13, cell surface associated C5 0.412 9q33-q34 complement component 5 NR4A2 0.412 2q22-q23 nuclear receptor subfamily 4, group A, member 2 EYS 0.411 6q12 eyes shut homolog (Drosophila) GPX2 0.406 14q24.1 glutathione peroxidase -

Gene Expression Patterns of the Female Genital Tract and Immunomodulation by Lactobacillus Species
Gene expression patterns of the female genital tract and immunomodulation by Lactobacillus species Andrea Gillian Abrahams ABRAND006 Supervisor: Dr Lindi Masson Co-Supervisors: Dr Arghavan Alisoltani-Dehkordi Dr Heather Jaspan Presented for Master of Science Degree (MSc) in Medical Virology Division of Medical Virology Faculty of Health Sciences University of Cape Town 23 September 2020 The copyright of this thesis vests in the author. No quotation from it or information derived from it is to be published without full acknowledgement of the source. The thesis is to be used for private study or non- commercial research purposes only. Published by the University of Cape Town (UCT) in terms of the non-exclusive license granted to UCT by the author. University of Cape Town Plagiarism Declaration “This thesis/dissertation has been submitted to the Turnitin module (or equivalent similarity and originality checking software) and I confirm that my supervisor has seen my report and any concerns revealed by such have been resolved with my supervisor.” Name: Andrea Gillian Abrahams Student number: ABRAND006 Signature: Date: 23 September 2020 i Acknowledgements First and foremost, I thank God for His grace and strength that has sustained me during this study and the completion of this thesis. To my supervisor, Dr Lindi Masson, I cannot thank you enough for all the support and encouragement you’ve given me these past two years. Thank you for your understanding and kindness during every challenge I’ve faced. You have taught me so much in this field and I am very grateful to you. A special thanks to my co-supervisor Dr Arghavan Alisoltani-Dehkordi for assistance in microarray data analysis training. -
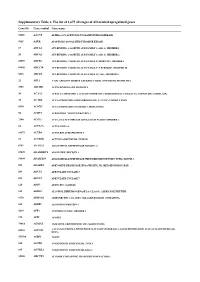
Supplementary Table 1. the List of 1,675 All Stages of AD-Related Upregulated Genes
Supplementary Table 1. The list of 1,675 all stages of AD-related upregulated genes Gene ID Gene symbol Gene name 51146 A4GNT ALPHA-1,4-N-ACETYLGLUCOSAMINYLTRANSFERASE 9625 AATK APOPTOSIS-ASSOCIATED TYROSINE KINASE 19 ABCA1 ATP-BINDING CASSETTE, SUB-FAMILY A (ABC1), MEMBER 1 20 ABCA2 ATP-BINDING CASSETTE, SUB-FAMILY A (ABC1), MEMBER 2 10058 ABCB6 ATP-BINDING CASSETTE, SUB-FAMILY B (MDR/TAP), MEMBER 6 89845 ABCC10 ATP-BINDING CASSETTE, SUB-FAMILY C (CFTR/MRP), MEMBER 10 5826 ABCD4 ATP-BINDING CASSETTE, SUB-FAMILY D (ALD), MEMBER 4 25 ABL1 V-ABL ABELSON MURINE LEUKEMIA VIRAL ONCOGENE HOMOLOG 1 3983 ABLIM1 ACTIN BINDING LIM PROTEIN 1 30 ACAA1 ACETYL-COENZYME A ACYLTRANSFERASE 1 (PEROXISOMAL 3-OXOACYL-COENZYME A THIOLASE) 35 ACADS ACYL-COENZYME A DEHYDROGENASE, C-2 TO C-3 SHORT CHAIN 8310 ACOX3 ACYL-COENZYME A OXIDASE 3, PRISTANOYL 56 ACRV1 ACROSOMAL VESICLE PROTEIN 1 2180 ACSL1 ACYL-COA SYNTHETASE LONG-CHAIN FAMILY MEMBER 1 86 ACTL6A ACTIN-LIKE 6A 93973 ACTR8 ACTIN-RELATED PROTEIN 8 91 ACVR1B ACTIVIN A RECEPTOR, TYPE IB 8747 ADAM21 ADAM METALLOPEPTIDASE DOMAIN 21 27299 ADAMDEC1 ADAM-LIKE, DECYSIN 1 56999 ADAMTS9 ADAM METALLOPEPTIDASE WITH THROMBOSPONDIN TYPE 1 MOTIF, 9 105 ADARB2 ADENOSINE DEAMINASE, RNA-SPECIFIC, B2 (RED2 HOMOLOG RAT) 109 ADCY3 ADENYLATE CYCLASE 3 113 ADCY7 ADENYLATE CYCLASE 7 120 ADD3 ADDUCIN 3 (GAMMA) 125 ADH1C ALCOHOL DEHYDROGENASE 1A (CLASS I), ALPHA POLYPEPTIDE 9370 ADIPOQ ADIPONECTIN, C1Q AND COLLAGEN DOMAIN CONTAINING 165 AEBP1 AE BINDING PROTEIN 1 4299 AFF1 AF4/FMR2 FAMILY, MEMBER 1 173 AFM AFAMIN 79814 AGMAT AGMATINE -

David Thesis 2007
The Role of Nuclear Factor-B in -Cell Survival and Function David Liuwantara A submission to the University of New South Wales in candidature for the degree of Doctor of Philosophy Gene Therapy and Autoimmunity Research Program Department of Inflammation and Immunology Garvan Institute of Medical Research Darlinghurst, Sydney, Australia November 2007 Statement of Originality ‘I hereby declare that this submission is my own work and to the best of my knowledge it contains no materials previously published or written by another person, or substantial proportions of material which have been accepted for the award of any other degree or diploma at UNSW or any other educational institution, except where due acknowledgement is made in the thesis. Any contribution made to the research by others, with whom I have worked at UNSW or elsewhere, is explicitly acknowledged in the thesis. I also declare that the intellectual content of this thesis is the product of my own work, except to the extent that assistance from others in the project’s design and conception or in style, presentation and linguistic expression is acknowleged.’ Signed …………………………………………………………… In Christ alone will I glory Though I could pride myself in battles won For I've been blessed beyond measure And by His strength alone I overcome Oh I could stop and count successes Like diamonds in my hands But those trophies could not equal To the grace by which I stand In Christ alone will I glory For only by His grace I am redeemed And only His tender mercy Could reach beyond my weakness to my need And now I seek no greater honor Than just to know Him more And to count my gains But losses to the glory of my Lord In Christ alone I place my trust And find my glory in the power of the cross In every victory let it be said of me My source of strength, my source of hope is Christ alone In Christ Alone by: Don Koch and Shawn Craig Paragon Music Corp © 1990 BMG Music Pty. -

The Crystal Structures of EDA-A1 and EDA-A2: Splice Variants with Distinct Receptor Specificity
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Structure, Vol. 11, 1513–1520, December, 2003, 2003 Elsevier Science Ltd. All rights reserved. DOI 10.1016/j.str.2003.11.009 The Crystal Structures of EDA-A1 and EDA-A2: Splice Variants with Distinct Receptor Specificity Sarah G. Hymowitz,1,* Deanne M. Compaan,1 tity with EDAR in their extracellular regions. Unlike some Minhong Yan,2 Heidi J.A. Wallweber,1 subgroups of the TNFL family, such as BAFF and APRIL, Vishva M. Dixit,2 Melissa A. Starovasnik,1 LT and TNF, or FasL, LIGHT, and TL1A (Bodmer et al., and Abraham M. de Vos1 2002; Migone et al., 2002), in which either the ligands 1Department of Protein Engineering or the receptors are promiscuous, EDA-A1 and EDA-A2 2 Department of Molecular Oncology are thus far known to interact only with EDAR and Genentech, Inc. XEDAR, respectively. 1 DNA Way The intracellular domains and signaling pathways of South San Francisco, California 94080 EDAR and XEDAR are also divergent, amplifying the switch between EDA-A1 and EDA-A2. EDAR possesses an intracellular death domain which interacts with the adaptor EDARDD, which in turn interacts with TRAFs 1, Summary 2, and 3 (Headon et al., 2001; Yan et al., 2002). XEDAR lacks a death domain and has been shown to signal via EDA is a tumor necrosis factor family member involved TRAF-6 (Yan et al., 2000; Naito et al., 2002; Sinha et al., in ectodermal development. Splice variants EDA-A1 2002).