Immunology: Questions and Answers
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Food Allergy Outline
Allergy Evaluation-What it all Means & Role of Allergist Sai R. Nimmagadda, M.D.. Associated Allergists and Asthma Specialists Ltd. Clinical Assistant Professor Of Pediatrics Northwestern University Chicago, Illinois Objectives of Presentation • Discuss the different options for allergy evaluation. – Skin tests – Immunocap Testing • Understand the results of Allergy testing in various allergic diseases. • Briefly Understand what an Allergist Does Common Allergic Diseases Seen in the Primary Care Office • Atopic Dermatitis/Eczema • Food Allergy • Allergic Rhinitis • Allergic Asthma • Allergic GI Diseases Factors that Influence Allergies Development and Expression Host Factors Environmental Factors . Genetic Indoor allergens - Atopy Outdoor allergens - Airway hyper Occupational sensitizers responsiveness Tobacco smoke . Gender Air Pollution . Obesity Respiratory Infections Diet © Global Initiative for Asthma Why Perform Allergy Testing? – Confirm Allergens and answer specific questions. • Am I allergic to my dog? • Do I have a milk allergy? • Have I outgrown my allergy? • Do I need medications? • Am I penicillin allergic? • Do I have a bee sting allergy Tests Performed in the Diagnostic Allergy Laboratory • Allergen-specific IgE (over 200 allergen extracts) – Pollen (weeds, grasses, trees), – Epidermal, dust mites, molds, – Foods, – Venoms, – Drugs, – Occupational allergens (e.g., natural rubber latex) • Total Serum IgE (anti-IgE; ABPA) • Multi-allergen screen for IgE antibody Diagnostic Allergy Testing Serological Confirmation of Sensitization History of RAST Testing • RAST (radioallergosorbent test) invented and marketed in 1974 • The suspected allergen is bound to an insoluble material and the patient's serum is added • If the serum contains antibodies to the allergen, those antibodies will bind to the allergen • Radiolabeled anti-human IgE antibody is added where it binds to those IgE antibodies already bound to the insoluble material • The unbound anti-human IgE antibodies are washed away. -
Syllabus for M
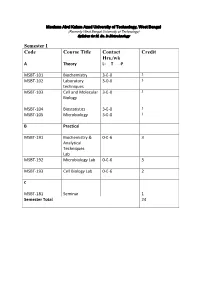
Maulana Abul Kalam Azad University of Technology, West Bengal (Formerly West Bengal University of Technology) Syllabus for M. Sc. In Biotechnology Semester I Code Course Title Contact Credit Hrs./wk A Theory L- T -P MSBT-101 Biochemistry 3-0-0 3 MSBT-102 Laboratory 3-0-0 3 techniques MSBT-103 Cell and Molecular 3-0-0 3 Biology MSBT-104 Biostatistics 3-0-0 3 MSBT-105 Microbiology 3-0-0 3 B Practical MSBT-191 Biochemistry & 0-0-6 3 Analytical Techniques Lab MSBT-192 Microbiology Lab 0-0-6 3 MSBT-193 Cell Biology Lab 0-0-6 2 C MSBT-181 Seminar 1 Semester Total 24 Maulana Abul Kalam Azad University of Technology, West Bengal (Formerly West Bengal University of Technology) Syllabus for M. Sc. In Biotechnology MSBT101: Biochemistry credits 3 Unit 1: Basic chemistry for biologists Formation of chemical bonds, molecular orbital (MO) theory and linear combination of atomic orbitals (LCAO),basics of mass spectrometry, molecules, Avogadro number, molarity, chemical reactions, reaction stoichiometry, rates of reaction, rate constants, order of reactions,kinetic versus thermodynamic controls of a reaction, reaction equilibrium (equilibrium constant); light and matter interactions (optical spectroscopy, fluorescence, bioluminescence, paramagnetism and diamagnetism, photoelectron spectroscopy; chemical bonds (ionic, covalent, Van derWalls forces); electronegativity, polarity; VSEPR theory and molecular geometry, dipole moment, orbital hybridizations; acids, bases and pH - Arrhenious theory, pH, ionic product of water, weak acids and bases, conjugate acid-base pairs, buffers and buffering action etc; chemical thermodynamics - internal energy, heat and temperature, enthalpy (bond enthalpy and reaction enthalpy), entropy, Gibbs free energy of ATP driven reactions, spontaneity versus driven reactions in biology;bond rotations and molecular conformations - Newman projections, conformational analysis of alkanes, alkenes and alkynes; functional groups, optically asymmetric carbon centers, amino acids, proteins, rotational freedoms in polypeptide backbone (Ramachandran plot). -

Technical Methods
J Clin Pathol 1987;40:581-588 J Clin Pathol: first published as 10.1136/jcp.40.5.581 on 1 May 1987. Downloaded from 56°C for 30 minutes. Technical methods Complement fixation tests were performed accord- ing to established methods,10 1 except that microtitre plates were used instead of World Health Organisation trays. For maximum sensitivity an ini- Cytomegalovirus (CMV) tial serum dilution of 1/4 was used. The antigen prep- antibody screening in blood aration used was a CMV complement fixation test antigen supplied by either Flow Laboratories Ltd, donors: modification of new latex Irvine, Scotland, or the Central Public Health Labo- ratory, Colindale, England. Guinea pig complements agglutination test compared with were supplied by Wellcome Diagnostics, Dartford, two standard methods England, or Don Whitly Scientific Ltd, Shipley, England. Complement fixation tests were performed A PUCKETT J E DAVIS From the Regional Blood using the following CMV antigen and complement Transfusion Centre, John Radeliffe Hospital, combinations: (1) PHLS CMV antigen + Wellcome Headington, Oxford, England Diagnostics complement, (2) PHLS CMV antigen + Don Whitly complement, and (3) Flow Laboratories CMV antigen + Wellcome Diagnostics complement. Infection with cytomegalovirus (CMV) is common, Immunofluorescence tests were performed and between 50 and 100% of adults may show evi- according to a standard method12 13 using substrate dence of infection.1 The transmission of the virus by slides of CMV infected (Westwood strain) fibroblasts blood transfusion2 and, therefore, the need to screen the Oxford Public Health Laboratory. donations intended for at risk groups such as provided by immunocompromised patients34 and neonates5 -7 iS CMV Scan passive latex agglutination kits were now well established. -

RAST Type Tests CPT: 86003
Medicare Local Coverage Determination Policy Allergy Testing RAST Type Tests CPT: 86003 Medically Supportive CMS Policy for Kentucky and Ohio ICD Codes are listed Local policies are determined by the performing test location. This is determined by the state on subsequent page(s) in which your performing laboratory resides and where your testing is commonly performed. of this document. Coverage Indications, Limitations, and/or Medical Necessity Radioallergosorbent test (RAST), fluoroallergosorbent test (FAST), and multiple antigen simultaneous tests are in vitro techniques for Add full policy information determining whether a patient's serum contains IgE antibodies against specific allergens of clinical importance. As with any allergy testing, the need for such tests is based on the findings during a complete history and physical examination of the patient. The multiple antigen simultaneous testing technique is similar to the RAST/FAST techniques in that it depends upon the existence of Template structure: allergic antibodies in the blood of the patient being tested. With the multiple antigen simultaneous test system, several antigens may be used to test for specific IgE simultaneously. First level is for headers such as limitations, ELISA (enzyme-linked immunosorbent assay) is another in vitro method of allergy testing for specific IgE antibodies against allergens. indications and usage guidelines This method is also a variation of RAST. Second level is for main body copy Limitations • Third level is for bullet (if needed) The following tests are considered to be not medically necessary and will be denied. • ELISA/Act qualitative antibody testing. This testing is used to determine in vitro reaction to various foods and relies on lymphocyte blastogenesis in response to certain food antigens. -

Test Directory
800.541.7891 509.755.8600 Test Directory Fax 509.921.7107 Billing Code Test Code [sunquest] (1,3)-BETA-D-GLUCAN (FUNGITELL) 13BGA 13BGA Synonyms Fungitell; Glucan Container Type Red top tube (plain) Store and Transport Refrigerated Specimen Type Serum Preferred Volume 2 mL Minimum Volume 0.5 mL Specimen Processing Separate serum from cells within 2 hours of collection and transfer to a standard PAML aliquot tube Room Temp Unacceptable Refrigerated 2 weeks Frozen (-20°C) 2 weeks Unacceptable Condition Hemolyzed, lipemic and icteric samples Reference Laboratory ARUP Reference Lab Test Code 2002434 CPT Codes 87449 Test Schedule Mon-Fri Turnaround Time 2-4 days Method Semi-Quantitative Colorimetry Test Includes (1,3-beta-D-glucan, pg/mL; (1,3-beta D-glucan Interpretation. Notes Reference ranges for pediatric patients (less than 18 years old) have not been established. Assay ranges were validated in adult subjects. Supply Item Number 1372 Billing Code Test Code [sunquest] 1, 5 ANHYDROGLUCITOL (GLYCOMARK) GLYMAR GLYMAR Synonyms Anhydroglucitol, 1,5 AD; GlycoMark® Container Type Serum separator tube (gold, brick, SST, or corvac) Store and Transport Refrigerated Specimen Type Serum Preferred Volume 1 mL Minimum Volume 0.2 mL Specimen Processing Allow serum specimen to clot completely at room temperature. Separate serum or plasma from cells and transfer to a standard PAML aliquot tube Room Temp 1 week Refrigerated 1 week Frozen (-20°C) 1 month Alternate Specimens Lavender (EDTA) Reference Laboratory ARUP Reference Lab Test Code 0081335 CPT Codes 84378 Test Schedule Mon-Fri Turnaround Time 2-4 days Method Quantitative Enzymatic Test Includes GlycoMark, ug/mL. -

Anti-Insulin Antibodies in Von Freien Und Totalen Anti-Insulin Dans Le Sérum Humain Human Serum Antikörpern in Humanserum
Février 2019 – Modèle 014 Cisbio Bioassays Parc Marcel Boiteux – BP 84175 – 30200 Codolet / France - Tél. 33 (0)4.66.79.67.00 ANTI-INSULIN AAI ANTIBODIES Trousse pour le dosage radioimmunologique Kit for radioimmunoassay for determination Kit zur radioimmunologischen Bestimmung des anticorps anti-insuline libres et totaux of free and total anti-insulin antibodies in von freien und totalen Anti-Insulin dans le sérum humain human serum Antikörpern in Humanserum La trousse contient : Kit content : Inhalt des Kits : Traceur ≤ 52 kBq 2 x 5 mL Tracer ≤ 52 kBq 2 x 5 mL Tracer ≤ 52 kBq 2 x 5 mL Solution précipitante 1 x 100 mL Precipitating solution 1 x 100 mL Präzipitationslösung 1 x 100 mL Réactif d’extraction 1 x 10 mL Extraction reagent 1 x 10 mL Extraktionsreagenz 1 x 10 mL Tampon de neutralisation 1 x 10 mL Neutralization buffer 1 x 10 mL Neutralisationspuffer 1 x 10 mL Contrôle négatif (C1) 1 x qsp 1 mL Negative control 1 x qs 1 mL Negativkontrolle 1 x qs 1 mL Contrôle positif (C2) 1 x qsp 1 mL Positive control 1 x qs 1 mL Positivkontrolle 1 x qs 1 mL Mode d’emploi 1 Instruction for use 1 Gebrauchsinformation 1 Attention : Certains réactifs contiennent de l’azoture de sodium Warning : Some reagents contain sodium azide Achtung : Einige Reagenzien enthalten Natriumazid Kit per il dosaggio radioimmunologico degli Equipo radioinmunológica para la Δοκιμασία για τον ραδιοανοσολογικό anticorpi anti-insulina liberi e totali nel siero determinación de los anticuerpos anti- προσδιορισμό των ελεύθερων και συνολικών umano insulina libres y totales en suero humano αντισωμάτων έναντι της ινσουλίνης στον ανθρώπινο ορό . -

Radioallergosorbent Test (RAST)—Reliable Tool Or Poor Substitute?
Radioallergosorbent test (RAST) VII Ö1 1 reliable tool or poor substitute? Edward W. Hein, M.D. An in vitro method, the radioallergosorbent test (RAST) has been developed for the detection of allergen-specific antibodies of the IgE class. Review of the literature shows that in comparison to skin testing, the RAST has a high degree of correlation (60% to 90% depending on the antigen); however, this method is not as sensitive as other tests (50% false-negative). The RAST is affected by blocking antibodies (IgG), resulting in false-negative values and high levels of IgE that bind on the allergen discs, giving false- positive findings. Because of these problems, RAST is somewhat limited for use in the clinical setting. Index terms: Allergy and immunology • Radioallergosor- bent test (RAST) Cleve Clin Q 50:361-366, Fall 1983 Skin testing has been the traditional method for diagnosis of IgE-mediated allergic disorders. This bioassay is highly sensitive, cost-effective, and safe when used by experienced personnel. In 1967, Wide et al,1 in Sweden, reported a new technique capable of detecting the minute quantities of allergen-specific IgE antibodies that circulate in the serum of allergic patients. This laboratory procedure, called RAST (radioallergosorbent test), utilized a solid- phase radioimmunoassay method. During the last decade this in vitro test has been refined and is now a commercially 1 Departments of Pediatric and Adolescent Medi- available laboratory test for clinical laboratories and, in kit cine, and Allergy and Immunology, The Cleveland form, for physicians' offices. Proponents of this new Clinic Foundation. (E.W.H., Head, Section Pediatric method claim that its results are more objective, safer for Allergy and Clinical Immunology.) Submitted for pub- lication May 1983; accepted June 1983. -

Hypersensitivity Reactions (Types I, II, III, IV)
Hypersensitivity Reactions (Types I, II, III, IV) April 15, 2009 Inflammatory response - local, eliminates antigen without extensively damaging the host’s tissue. Hypersensitivity - immune & inflammatory responses that are harmful to the host (von Pirquet, 1906) - Type I Produce effector molecules Capable of ingesting foreign Particles Association with parasite infection Modified from Abbas, Lichtman & Pillai, Table 19-1 Type I hypersensitivity response IgE VH V L Cε1 CL Binds to mast cell Normal serum level = 0.0003 mg/ml Binds Fc region of IgE Link Intracellular signal trans. Initiation of degranulation Larche et al. Nat. Rev. Immunol 6:761-771, 2006 Abbas, Lichtman & Pillai,19-8 Factors in the development of allergic diseases • Geographical distribution • Environmental factors - climate, air pollution, socioeconomic status • Genetic risk factors • “Hygiene hypothesis” – Older siblings, day care – Exposure to certain foods, farm animals – Exposure to antibiotics during infancy • Cytokine milieu Adapted from Bach, JF. N Engl J Med 347:911, 2002. Upham & Holt. Curr Opin Allergy Clin Immunol 5:167, 2005 Also: Papadopoulos and Kalobatsou. Curr Op Allergy Clin Immunol 7:91-95, 2007 IgE-mediated diseases in humans • Systemic (anaphylactic shock) •Asthma – Classification by immunopathological phenotype can be used to determine management strategies • Hay fever (allergic rhinitis) • Allergic conjunctivitis • Skin reactions • Food allergies Diseases in Humans (I) • Systemic anaphylaxis - potentially fatal - due to food ingestion (eggs, shellfish, -

BLOOD TYPE) Methodology: Tube Agglutination BBK Set Up: Daily, As Ordered ABORH BLOOD TYPE 6.0 Ml Whole Blood (Pink) ABORH Report Available: Same Day
LAB OE TEST REFERENCE SPECIMEN ORDER ORDER PROCEDURE RANGE REQUIREMENTS MNEMONIC NAME ABORH GROUP (BLOOD TYPE) Methodology: Tube agglutination BBK Set up: Daily, as ordered ABORH BLOOD TYPE 6.0 mL whole blood (Pink) ABORH Report available: Same day CPT Code: 86900, 86901 ACA or ACLA - See Anti-Cardiolipin Antibodies ACE - see Angiotensin-1 Converting Enzyme ACETAMINOPHEN, SERUM Methodology: Immunoassay 1 mL blood (Gn -Li (PST)) Set up: Daily, as ordered or LAB ACETAMINOPHEN Accompanies report Report available: Same day 1 mL serum (SS) ACET Minimum: 0.5 mL CPT Code: 80329 ACETYLCHOLINE RECEPTOR 1.0 mL serum (SS) BINDING ANTIBODIES (QUEST 206) Minimum: 0.5 mL ACETYLCHOLINE BINDING Methodology: RIA LAB Accompanies report RECEP Set up: Tues-Sat Allow serum to clot at room ACETYL BIND Report available: 1-2 days temperature. Serum should be separated from cells within 1 CPT Code: 83519 hour of collection. ACETYLCHOLINE RECEPTOR BLOCKING ANTIBODIES (QUEST 34459) 1.0 mL serum (SS) centrifuge ACETYLCHOLINE Methodology: RIA with 1 hr of collection LAB Accompanies report BLOCKING RECEP Set up:Mon, Wed, Fri ACETYL BLO Report available: Next day Minimum:0.5 mL CPT Code: 83519 ACETYLCHOLINE RECEPTORMODULATING ANTIBODY (QUEST 26474) 1 mL serum (SS) ACETYLCHOL LAB Methodology: RIA Accompanies report MODULATING RECEP ACETYL MOD Set up: Tue,Thur,Sun Minimum: 0.5 mL Report available: 5 days CPT Code: 83519 ACETYLCHOLINESTERASE, QUALITATIVE, GEL ELECTROPHORESIS (QUEST 185314) This test is automatically performed on all 1.5 mL Amniotic fluid, ROOM Alpha-Fetroprotein -

Serological Methods in the Identification and Characterization of Viruses
CHAPTER 4 Serological Methods in the Identification and Characterization of Viruses M. H. V. Van Regenmortel Laboratoire de Virologie Institut de Biologie Mo!eculaire et Cellulaire 67000 Strasbourg, France 1. INTRODUCTION The purpose of this chapter is to present an integrated view of the various serological techniques that have been used in virology. The accent will be placed on the principles that govern each type of test and on the general applicability of the different serological techniques in all fields of virus research. In recent years, advances in serological tech niques have sometimes been applied in only one area of virology, although they could have been equally useful to workers studying other groups of viruses. No doubt this stems from the host-oriented approach that has guided the compartmentation of virology into separate fields of specialization. When it comes to serological properties, however, the similarities between animal, insect, bacterial, and plant viruses are paramount. The same immunochemical principles govern the in vitro serological reactions of all viral antigens, and much of general interest can be learned from the findings obtained with each particular group of viruses. An attempt will be made here to emphasize the general validity of specific experimental procedures. A number of recent reviews restricted to the serology of particular groups of viruses are available 183 H. Fraenkel-Conrat et al. (eds.), Comprehensive Virology © Plenum Press, New York 1981 184 Chapter 4 (Cowan, 1973; Schmidt and Lennette, 1973; Ball, 1974; Kurstak and Morisset, 1974; Burns and Allison, 1975; Mazzone and Tignor, 1976; Mayr et al., 1977; Tyrrell, 1978; Van Regenmortel, 1978; Cooper, 1979). -

Msc Biochemistry Autonomy 2015-17
Bhavan’s Vivekananda College of Science, Humanities and Commerce, Sainikpuri, Secunderabad–500094 Autonomous (Accredited with ‘A’ grade by NAAC) MSc Biochemistry Autonomy 2015-17 Bhavan’s Vivekananda College of Science, Humanities and Commerce, Sainikpuri, Secunderabad–500094 Autonomous (Accredited with ‘A’ grade by NAAC) M.Sc. Biochemistry Syllabus (Effective from 2015 admitted batch) SEMESTER I PAPERS TITLE Teaching Credits Internal Final hrs/week marks exam marks 1 Paper-I : BI101T:Chemistry and Metabolism of Proteins, Lipids and Porphyrins 4 4 30 70 2 Paper-II : BI102T:Chemistry and Metabolism of Carbohydrates, Vitamins 4 4 30 70 and Nucleic Acids 3 Paper-III: BI 103T: Bio-Analytical Techniques 4 4 30 70 4 Paper-IV: BI104T:BioenergeticsAndCellBiology 4 4 30 70 5 Paper-V: BI105P: Amino acids and protein analysis 8 4 -- 100 6 Paper-VI: BI106P: Carbohydrate and lipid analysis 8 4 -- 100 Total 32 24 120 480 SEMESTER II PAPERS TITLE Teaching Credits Internal Final hrs/week marks exam marks 1 Paper-I:BI201T:Enzymology 4 4 30 70 2 Paper-II:BI202T:MolecularBiology 4 4 30 70 3 Paper-III:BI203T:BiochemicalGeneticsAndModelOrganisms 4 4 30 70 4 Paper-IV: B1 204T: Computational methods and Cell study methods 4 4 30 70 5 Paper-V:BI205P:Enzymology and Biochemical preparations 8 4 -- 100 6 Paper-VI:BI206P: Molecular Biology, Genetics and Quantitative Biology 8 4 -- 100 Total 32 24 120 480 SEMESTER III PAPERS TITLE Teaching Credits Internal Final hrs/week marks exam marks 1 Paper-I:BI301T:GeneRegulation and Genetic Engineering 4 4 30 70 2 Paper-II:BI302T:Immunology and Immunotechnology 4 4 30 70 3 Paper-III:BI303T: Virology, Nutrition & Clinical Biochemistry. -

KPNW Specimen Requirements ***Please Hit 'Ctrl'+'F' to Open the 'Find on This Page' Funtion
Page 1 of 368 KPNW Specimen Requirements ***Please hit 'Ctrl'+'F' to open the 'Find on this page' funtion. Panel Name Panel Description Preferred Collection/Volume: RED 10 No Gel Barrier Tubes Volume: 1.0 mL Serum Alternative Collection: GRN Li Hep or LAV EDTA Volume: 1.0 mL Plasma TAT: Report available: 5 Days Test Schedule: Saturday Morning Method: Liquid Chromatography/Tandem Mass Spectrometry Test Facility: Quest Diagnostics Nichols Inst San Juan Capistrano 33608 Ortega Highway San Juan Capistrano, CA 92690-6130 Clinical Data: 11-Deoxycortisol (Compound S) is useful in diagnosing patients with 11-beta- hydroxylase deficiency (second leading cause of congenital adrenal hyperplasia) and 11-Deoxycortisol primary (adrenal failure) or secondary (hypothalmic-pituitary ACTH deficiency) adrenal insufficiency. Patient Preparation: An early morning specimen is preferred Specimen Stability: Room Temperature: 4 days Refrigerated: 4 days Frozen: 4 weeks Ship serum frozen or refrigerated Test Code: CHANTILLY T.C.30543 TO SJC TC 30543X Processing: Centrifuge and aliquot into Referred Tests aliquot tube. Note if serum or plasma on specimen. Freeze solid. Transport: Transport frozen on ice. Preferred Collection/Volume: Preserve 24-hour urine with 25 mL of 50% Acetic Acid (preferred) or 30 mL 6N HCl or 1 gram Boric Acid/100 mL (acceptable) during collection. Refrigerate during collection. TAT: Next Day Test Schedule: Monday-Friday Night Method: Colorimetric Test Facility: Quest Diagnostics Nichols Institute 14225 Newbrook Drive Chantilly, VA 20153 17 Ketosteroids Total Pediatric 24 Hr Urine Labeling: Please specify on the request form and on the urine container the patient's age, sex, and (Includes Creatinine the total 24-hour urine volume Ratio) Specimen Stability: Room Temperature: 8 hours Refrigerated: 7 days Frozen: 30 days (-20c) Please specify on the request form and on the urine container the patient's age, sex, and the total 24-hour urine volume.