Complement Recognition Pathways in Renal Transplantation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Activation Regulates Alternative Pathway Complement Necrotic
Properdin Binds to Late Apoptotic and Necrotic Cells Independently of C3b and Regulates Alternative Pathway Complement Activation This information is current as of September 27, 2021. Wei Xu, Stefan P. Berger, Leendert A. Trouw, Hetty C. de Boer, Nicole Schlagwein, Chantal Mutsaers, Mohamed R. Daha and Cees van Kooten J Immunol 2008; 180:7613-7621; ; doi: 10.4049/jimmunol.180.11.7613 Downloaded from http://www.jimmunol.org/content/180/11/7613 References This article cites 56 articles, 27 of which you can access for free at: http://www.jimmunol.org/ http://www.jimmunol.org/content/180/11/7613.full#ref-list-1 Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists by guest on September 27, 2021 • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2008 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Properdin Binds to Late Apoptotic and Necrotic Cells Independently of C3b and Regulates Alternative Pathway Complement Activation1,2 Wei Xu,* Stefan P. Berger,* Leendert A. -

An Anticomplement Agent That Homes to the Damaged Brain and Promotes Recovery After Traumatic Brain Injury in Mice
An anticomplement agent that homes to the damaged brain and promotes recovery after traumatic brain injury in mice Marieta M. Rusevaa,1,2, Valeria Ramagliab,1, B. Paul Morgana, and Claire L. Harrisa,3 aInstitute of Infection and Immunity, School of Medicine, Cardiff University, Cardiff CF14 4XN, United Kingdom; and bDepartment of Genome Analysis, Academic Medical Center, Amsterdam 1105 AZ, The Netherlands Edited by Douglas T. Fearon, Cornell University, Cambridge, United Kingdom, and approved September 29, 2015 (received for review July 15, 2015) Activation of complement is a key determinant of neuropathology to rapidly and specifically inhibit MAC at sites of complement and disability after traumatic brain injury (TBI), and inhibition is activation, and test its therapeutic potential in experimental TBI. neuroprotective. However, systemic complement is essential to The construct, termed CD59-2a-CRIg, comprises CD59a linked fight infections, a critical complication of TBI. We describe a to CRIg via the murine IgG2a hinge. CD59a prevents assembly targeted complement inhibitor, comprising complement receptor of MAC in cell membranes (16), whereas CRIg binds C3b/iC3b of the Ig superfamily (CRIg) fused with complement regulator CD59a, deposited at sites of complement activation (17). The IgG2a designed to inhibit membrane attack complex (MAC) assembly at hinge promotes dimerization to increase ligand avidity. CD59- sites of C3b/iC3b deposition. CRIg and CD59a were linked via the 2a-CRIg protected in the TBI model, demonstrating that site- IgG2a hinge, yielding CD59-2a-CRIg dimer with increased iC3b/C3b targeted anti-MAC therapeutics may be effective in prevention binding avidity and MAC inhibitory activity. CD59-2a-CRIg inhibited of secondary neuropathology and improve neurologic recovery MAC formation and prevented complement-mediated lysis in vitro. -

Supplementary Table 1: Adhesion Genes Data Set
Supplementary Table 1: Adhesion genes data set PROBE Entrez Gene ID Celera Gene ID Gene_Symbol Gene_Name 160832 1 hCG201364.3 A1BG alpha-1-B glycoprotein 223658 1 hCG201364.3 A1BG alpha-1-B glycoprotein 212988 102 hCG40040.3 ADAM10 ADAM metallopeptidase domain 10 133411 4185 hCG28232.2 ADAM11 ADAM metallopeptidase domain 11 110695 8038 hCG40937.4 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 195222 8038 hCG40937.4 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 165344 8751 hCG20021.3 ADAM15 ADAM metallopeptidase domain 15 (metargidin) 189065 6868 null ADAM17 ADAM metallopeptidase domain 17 (tumor necrosis factor, alpha, converting enzyme) 108119 8728 hCG15398.4 ADAM19 ADAM metallopeptidase domain 19 (meltrin beta) 117763 8748 hCG20675.3 ADAM20 ADAM metallopeptidase domain 20 126448 8747 hCG1785634.2 ADAM21 ADAM metallopeptidase domain 21 208981 8747 hCG1785634.2|hCG2042897 ADAM21 ADAM metallopeptidase domain 21 180903 53616 hCG17212.4 ADAM22 ADAM metallopeptidase domain 22 177272 8745 hCG1811623.1 ADAM23 ADAM metallopeptidase domain 23 102384 10863 hCG1818505.1 ADAM28 ADAM metallopeptidase domain 28 119968 11086 hCG1786734.2 ADAM29 ADAM metallopeptidase domain 29 205542 11085 hCG1997196.1 ADAM30 ADAM metallopeptidase domain 30 148417 80332 hCG39255.4 ADAM33 ADAM metallopeptidase domain 33 140492 8756 hCG1789002.2 ADAM7 ADAM metallopeptidase domain 7 122603 101 hCG1816947.1 ADAM8 ADAM metallopeptidase domain 8 183965 8754 hCG1996391 ADAM9 ADAM metallopeptidase domain 9 (meltrin gamma) 129974 27299 hCG15447.3 ADAMDEC1 ADAM-like, -

Complement Inactivation Strategy of Staphylococcus Aureus Using Decay-Accelerating Factor and the Response of Infected Hacat Cells
International Journal of Molecular Sciences Article Complement Inactivation Strategy of Staphylococcus aureus Using Decay-Accelerating Factor and the Response of Infected HaCaT Cells Kyoung Ok Jang 1, Youn Woo Lee 1, Hangeun Kim 2,* and Dae Kyun Chung 1,2,3,* 1 Graduate School of Biotechnology, Kyung Hee University, Yongin 17104, Korea; [email protected] (K.O.J.); [email protected] (Y.W.L.) 2 Research and Development Center, Skin Biotechnology Center Inc., Yongin 17104, Korea 3 Skin Biotechnology Center, Kyung Hee University, Suwon 16229, Korea * Correspondence: [email protected] (H.K.); [email protected] (D.K.C.); Tel.: +82-31-201-2487 (H.K.); +82-31-888-6170 (D.K.C.) Abstract: Staphylococcus aureus is a species of Gram-positive staphylococcus. It can cause sinusitis, respiratory infections, skin infections, and food poisoning. Recently, it was discovered that S. aureus infects epithelial cells, but the interaction between S. aureus and the host is not well known. In this study, we confirmed S. aureus to be internalized by HaCaT cells using the ESAT-6-like protein EsxB and amplified within the host over time by escaping host immunity. S. aureus increases the expression of decay-accelerating factor (CD55) on the surfaces of host cells, which inhibits the activation of the complement system. This mechanism makes it possible for S. aureus to survive in host cells. S. aureus, sufficiently amplified within the host, is released through the initiation of cell death. On the other hand, the infected host cells increase their surface expression of UL16 binding protein 1 to inform Citation: Jang, K.O.; Lee, Y.W.; Kim, immune cells that they are infected and try to be eliminated. -

The Complement System: a Powerful Modulator and Effector of Astrocyte Function in the Healthy and Diseased Central Nervous System
cells Review The Complement System: A Powerful Modulator and Effector of Astrocyte Function in the Healthy and Diseased Central Nervous System Marcela Pekna 1,3,4,* and Milos Pekny 2,3,4 1 Laboratory of Regenerative Neuroimmunology, Center for Brain Repair, Department of Clinical Neuroscience, Institute of Neuroscience and Physiology, Sahlgrenska Academy at the University of Gothenburg, 40530 Gothenburg, Sweden 2 Laboratory of Astrocyte Biology and CNS Regeneration, Center for Brain Repair, Department of Clinical Neuroscience, Institute of Neuroscience and Physiology, Sahlgrenska Academy at the University of Gothenburg, 40530 Gothenburg, Sweden; [email protected] 3 Florey Institute of Neuroscience and Mental Health, Parkville, Melbourne 3010, Australia 4 School of Medicine and Public Health, University of Newcastle, Newcastle 2308, Australia * Correspondence: [email protected]; Tel.: +46-31-786-3581 Abstract: The complement system, an effector arm of the innate immune system that plays a critical role in tissue inflammation, the elimination of pathogens and the clearance of dead cells and cell debris, has emerged as a regulator of many processes in the central nervous system, including neural cell genesis and migration, control of synapse number and function, and modulation of glial cell responses. Complement dysfunction has also been put forward as a major contributor to neurological disease. Astrocytes are neuroectoderm-derived glial cells that maintain water and ionic homeostasis, and control cerebral blood flow and multiple aspects of neuronal functioning. By virtue of their Citation: Pekna, M.; Pekny, M. The Complement System: A Powerful expression of soluble as well as membrane-bound complement proteins and receptors, astrocytes are Modulator and Effector of Astrocyte able to both send and receive complement-related signals. -

Recognition of Microbial Glycans by Soluble Human Lectins
Available online at www.sciencedirect.com ScienceDirect Recognition of microbial glycans by soluble human lectins 3 1 1,2 Darryl A Wesener , Amanda Dugan and Laura L Kiessling Human innate immune lectins that recognize microbial glycans implicated in the regulation of microbial colonization and can conduct microbial surveillance and thereby help prevent in protection against infection. Seminal research on the infection. Structural analysis of soluble lectins has provided acute response to bacterial infection led to the identifica- invaluable insight into how these proteins recognize their tion of secreted factors that include C-reactive protein cognate carbohydrate ligands and how this recognition gives (CRP) and mannose-binding lectin (MBL) [1,3]. Both rise to biological function. In this opinion, we cover the CRP and MBL can recognize carbohydrate antigens on structural features of lectins that allow them to mediate the surface of pathogens, including Streptococcus pneumo- microbial recognition, highlighting examples from the collectin, niae and Staphylococcus aureus and then promote comple- Reg protein, galectin, pentraxin, ficolin and intelectin families. ment-mediated opsonization and cell killing [4]. Since These analyses reveal how some lectins (e.g., human intelectin- these initial observations, other lectins have been impli- 1) can recognize glycan epitopes that are remarkably diverse, cated in microbial recognition. Like MBL some of these yet still differentiate between mammalian and microbial proteins are C-type lectins, while others are members of glycans. We additionally discuss strategies to identify lectins the ficolin, pentraxin, galectin, or intelectin families. that recognize microbial glycans and highlight tools that Many of the lectins that function in microbial surveillance facilitate these discovery efforts. -
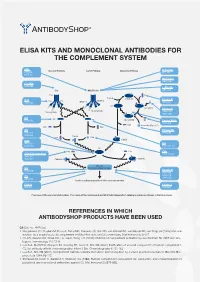
Elisa Kits and Monoclonal Antibodies for the Complement System
ELISA KITS AND MONOCLONAL ANTIBODIES FOR THE COMPLEMENT SYSTEM MBL ClassicalÊPathway LectinÊPathway AlternativeÊPathway H-Ficolin KITÊ029 RIGÊ334 HYBÊ131 M-Ficolin ABSÊ036 C1-INH HYBÊ288 L-Ficolin C1q MBL/Ficolin ABSÊ005 C1q B H2O C3Ê(H2O) FactorÊB C1s C1s C1r MASP-2 D ABSÊ002 MASP-1 HAVÊ005 C3 C3Ê(H O)ÊB 2 FactorÊD Carbohydrate Carbohydrate GAUÊ010 GAUÊ008 C2 C2 C4 C3Ê(H O)ÊBb HYBÊ050 2 Ba FactorÊBa/b HYBÊ008 C3a ProperdinÊ(FactorÊP) C2b C4a C3 Properdin HAVÊ004 HYBÊ0393 HYBÊ005 C4b2a C2a C4b C3bBb C3d HAVÊ003 C4 HYBÊ030 HYBÊ162-04 C3 C3a C3a C4b C3a/C3aÊ(desArg) HYBÊ162-02 GAUÊ013 GAUÊ017 C4b2a3b C3bBb3b C5Ê-ÊC9 C5 MembraneÊattackÊcomplex HYBÊ029 FactorÊH HYBÊ268 GAUÊ018 C9 GAUÊ020 ABSÊ004 LysisÊorÊphagocytosisÊofÊtheÊmicroorganism C5b-9 FactorÊI DIAÊ011 HYBÊ061 OverviewÊofÊtheÊcomplementÊsystem.ÊTheÊnameÊofÊtheÊcomponentÊandÊBioPortoÊDiagnostics'ÊcatalogÊnumberÊareÊshownÊinÊtheÊblueÊboxes. REFERENCES IN WHICH ANTIBODYSHOP PRODUCTS HAVE BEEN USED C2 Cat. no. HYB 050 Rooijakkers SH, Ruyken M, Roos A, Daha MR, Presanis JS, Sim RB, van Wamel WJ, van Kessel KP, van Strijp JA (2005) Immune evasion by a staphylococcal complement inhibitor that acts on C3 convertases. Nat Immunol 6:920-7. Oh KS, Kweon MH, Rhee KH, Ho Lee K, Sung HC (2003) Inhibition of complement activation by recombinant Sh-CRIT-ed1 ana- logues. Immunology 110:73-9. Laich A, Moffatt B, Wong KHN, Hickling TP, Koch C, Sim RB (2001) Purifi cation of second component of human complement, C2, by antibody affi nity chromatography. Intern J Bio-Chromatography 6:151-162. Laich A, Sim RB (2001) Complement C4bC2 complex formation: an investigation by surface plasmon resonance. Biochim Bio- phys Acta 1544:96-112. Stenbaek EI, Koch C, Barkholt V, Welinder KG (1986) Human complement component C2: production and characterization of polyclonal and monoclonal antibodies against C2. -

Table 1. Swine Proteins Identified As Differentially Expressed at 24Dpi in OURT 88/3 Infected Animals
Table 1. swine proteins identified as differentially expressed at 24dpi in OURT 88/3 infected animals. Gene name Protein ID Protein Name -Log p-value control vs A_24DPI Difference control Vs A_24DPI F8 K7GL28 Coagulation factor VIII 2.123919902 5.42533493 PPBP F1RUL6 C-X-C motif chemokine 3.219079808 4.493174871 SDPR I3LDR9 Caveolae associated protein 2 2.191007299 4.085711161 IGHG L8B0X5 IgG heavy chain 2.084611488 -4.282530149 LOC100517145 F1S3H9 Complement C3 (LOC100517145) 3.885740476 -4.364484406 GOLM1 F1S4I1 Golgi membrane protein 1 1.746130664 -4.767168681 FCN2 I3L5W3 Ficolin-2 2.937884686 -6.029483795 Table 2. swine proteins identified as differentially expressed at 7dpi in Benin ΔMGF infected animals. Gene name Protein ID Protein Name -Log p-value control vs B_7DPI Difference control Vs B_7DPI A0A075B7I5 Ig-like domain-containing protein 1.765578164 -3.480728149 ATP5A1 F1RPS8_PIG ATP synthase subunit alpha 2.270386995 3.270935059 LOC100627396 F1RX35_PIG Fibrinogen C-terminal domain-containing protein 2.211242648 3.967363358 LOC100514666;LOC102158263 F1RX36_PIG Fibrinogen alpha chain 2.337934993 3.758180618 FGB F1RX37_PIG Fibrinogen beta chain 2.411948004 4.03753376 PSMA8 F1SBA5_PIG Proteasome subunit alpha type 1.473601007 -3.815182686 ACAN F1SKR0_PIG Aggrecan core protein 1.974489764 -3.726634026 TFG F1SL01_PIG PB1 domain-containing protein 1.809215274 -3.131304741 LOC100154408 F1SSL6_PIG Proteasome subunit alpha type 1.701949053 -3.944885254 PSMA4 F2Z528_PIG Proteasome subunit alpha type 2.045768185 -4.502977371 PSMA5 F2Z5K2_PIG -

Complement Receptor CD46 Co-Stimulates Optimal Human CD8 T
ARTICLE DOI: 10.1038/s41467-018-06706-z OPEN Complement receptor CD46 co-stimulates optimal human CD8+ T cell effector function via fatty acid metabolism Giuseppina Arbore1,2, Erin E. West3, Jubayer Rahman3, Gaelle Le Friec2, Nathalie Niyonzima3,4, Mehdi Pirooznia 3, Ilker Tunc3, Polychronis Pavlidis2, Nicholas Powell2, Yuesheng Li3, Poching Liu3, Aude Servais5, Lionel Couzi6, Veronique Fremeaux-Bacchi7, Leo Placais3, Alastair Ferraro8, Patrick R. Walsh9, David Kavanagh9, Behdad Afzali 3,10, Paul Lavender2, Helen J. Lachmann11 & Claudia Kemper2,3,12 1234567890():,; The induction of human CD4+ Th1 cells requires autocrine stimulation of the complement receptor CD46 in direct crosstalk with a CD4+ T cell-intrinsic NLRP3 inflammasome. However, it is unclear whether human cytotoxic CD8+ T cell (CTL) responses also rely on an intrinsic complement-inflammasome axis. Here we show, using CTLs from patients with CD46 deficiency or with constitutively-active NLRP3, that CD46 delivers co-stimulatory signals for optimal CTL activity by augmenting nutrient-influx and fatty acid synthesis. Sur- prisingly, although CTLs express NLRP3, a canonical NLRP3 inflammasome is not required for normal human CTL activity, as CTLs from patients with hyperactive NLRP3 activity function normally. These findings establish autocrine complement and CD46 activity as integral components of normal human CTL biology, and, since CD46 is only present in humans, emphasize the divergent roles of innate immune sensors between mice and men. 1 Division of Immunology, Transplantation and Infectious Diseases, San Raffaele Scientific Institute, Milano, Italy. 2 School of Immunology and Microbial Sciences, King’s College London, London, UK. 3 Laboratory of Molecular Immunology and the Immunology Center, National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health (NIH), Bethesda, MD, USA. -

Complement Receptor 1 Therapeutics for Prevention of Immune Hemolysis
Review: complement receptor 1 therapeutics for prevention of immune hemolysis K.YAZDANBAKHSH The complement system plays a crucial role in fighting infections biological activities, it has to be activated. Activation and is an important link between the innate and adaptive immune occurs in a sequence that involves proteolytic cleavage responses. However, inappropriate complement activation can cause tissue damage, and it underlies the pathology of many of the complement components, resulting in the diseases. In the transfusion medicine setting, complement release of active biological mediators and the assembly sensitization of RBCs can lead to both intravascular and of active enzyme molecules that result in cleavage of extravascular destruction. Moreover, complement deficiencies are 1 associated with autoimmune disorders, including autoimmune the next downstream complement component. hemolytic anemia (AIHA). Complement receptor 1 (CR1) is a large Depending on the nature of the activators, three single-pass glycoprotein that is expressed on a variety of cell types complement activation pathways have been described: in blood, including RBCs and immune cells. Among its multiple the antibody-dependent classical pathway and the functions is its ability to inhibit complement activation. Furthermore, gene knockout studies in mice implicate a role for antibody-independent alternative and lectin pathways CR1 (along with the alternatively spliced gene product CR2) in (Fig. 1).1 Common to all three pathways are two prevention of autoimmunity. This review discusses the possibility critical steps: the assembly of the C3 convertase that the CR1 protein may be manipulated to prevent and treat AIHA. In addition, it will be shown in an in vivo mouse model of enzymes and the activation of C5 convertases. -

C1q and Mannose-Binding Lectin Interact with CR1 in The
C1q and Mannose-Binding Lectin Interact with CR1 in the Same Region on CCP24-25 Modules Mickaël Jacquet, Gianluca Cioci, Guillaume Fouet, Isabelle Bally, Nicole Thielens, Christine Gaboriaud, Véronique Rossi To cite this version: Mickaël Jacquet, Gianluca Cioci, Guillaume Fouet, Isabelle Bally, Nicole Thielens, et al.. C1q and Mannose-Binding Lectin Interact with CR1 in the Same Region on CCP24-25 Modules. Frontiers in Immunology, Frontiers, 2018, 9 (453), 11 p. 10.3389/fimmu.2018.00453. hal-01730166 HAL Id: hal-01730166 https://hal.univ-grenoble-alpes.fr/hal-01730166 Submitted on 5 Dec 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution| 4.0 International License ORIGINAL RESEARCH published: 07 March 2018 doi: 10.3389/fimmu.2018.00453 C1q and Mannose-Binding Lectin Interact with CR1 in the Same Region on CCP24-25 Modules Mickaël Jacquet, Gianluca Cioci, Guillaume Fouet, Isabelle Bally, Nicole M. Thielens, Christine Gaboriaud and Véronique Rossi* Univ. Grenoble Alpes, CEA, CNRS, IBS, Grenoble, France Complement receptor type 1 (CR1) is a multi modular membrane receptor composed of 30 homologous complement control protein modules (CCP) organized in four different functional regions called long homologous repeats (LHR A, B, C, and D). -

Targeting of Mannan-Binding Lectin-Associated Serine Protease-2 Confers Protection from Myocardial and Gastrointestinal Ischemia/Reperfusion Injury
Targeting of mannan-binding lectin-associated serine protease-2 confers protection from myocardial and gastrointestinal ischemia/reperfusion injury Wilhelm J. Schwaeblea,1, Nicholas J. Lyncha, James E. Clarkb, Michael Marberb, Nilesh J. Samanic, Youssif Mohammed Alia,d, Thomas Dudlere, Brian Parente, Karl Lhottaf, Russell Wallisa, Conrad A. Farrarg, Steven Sacksg, Haekyung Leeh, Ming Zhangh, Daisuke Iwakii, Minoru Takahashii, Teizo Fujitai, Clark E. Tedforde, and Cordula M. Stovera Departments of aInfection, Immunity, and Inflammation and cCardiovascular Sciences, University of Leicester, Leicester LE1 9HN, United Kingdom; bBritish Heart Foundation Centre and gMedical Research Council Centre for Transplantation and National Institute for Health Research Biomedical Research Centre at Guy’s and St. Thomas’ National Health Service Foundation Trust, King’s College London, London SE1 9RT, United Kingdom; dFaculty of Pharmacy, Department of Microbiology, University of Mansoura, Mansoura 35516, Egypt; eOmeros Corporation, Seattle, WA 98101; fLandeskrankenhaus Feldkirch, 6807 Feldkirch, Austria; hDepartment of Anesthesiology, State University of New York-Downstate Medical Center, New York, NY 11203; and iDepartment of Immunology, Fukushima Medical University, Fukushima 960-1295, Japan Edited* by Douglas T. Fearon, University of Cambridge School of Clinical Medicine, Cambridge, United Kingdom, and approved March 16, 2011 (received for review February 1, 2011) Complement research experienced a renaissance with the discovery aberrant glycosylation