Activation Regulates Alternative Pathway Complement Necrotic
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

C3b Catalog Number: A114 Sizes Available
Name: C3b Catalog Number: A114 Sizes Available: 250 µg/vial Concentration: 1.0 mg/mL (see Certificate of Analysis for actual concentration) Form: Liquid Purity: >90% by SDS-PAGE Buffer: 10 mM sodium phosphate, 145 mM NaCl, pH 7.3 Extinction Coeff. A280 nm = 1.03 at 1.0 mg/mL Molecular Weight: 176,000 Da (2 chains) Preservative: None, 0.22 µm filtered Storage: -70oC or below. Avoid freeze/thaw. Source: Normal human serum (shown by certified tests to be negative for HBsAg and for antibodies to HCV, HIV-1 and HIV-II). Precautions: Use normal precautions for handling human blood products. Origin: Manufactured in the USA. General Description C3b is derived from native C3 upon cleavage and release of C3a. It is prepared by cleavage with the alternative pathway C3 convertase. This is important because only a single bond in C3 is cleaved by the native convertase, while cleavage by other proteases, such as trypsin, results in multiple cleavages at many other sites in the protein. Native human C3b is a glycosylated (~2.8%) polypeptide containing two disulfide-linked chains. C3b is central to the function of all three pathways of complement (Law, S.K.A. and Reid, K.B.M. (1995)). Initiation of each pathway generates proteolytic enzyme complexes (C3 convertases) which are bound to the target surface. These enzymes cleave a peptide bond in C3 releasing the anaphylatoxin C3a and activating C3b. For a brief time (~60 µs) this nascent C3b is capable of reacting with and covalently coupling to hydroxyl groups on the target surface. -

Complement Recognition Pathways in Renal Transplantation
BRIEF REVIEW www.jasn.org Complement Recognition Pathways in Renal Transplantation Christopher L. Nauser, Conrad A. Farrar, and Steven H. Sacks Medical Research Council Centre for Transplantation, Division of Transplantation Immunology and Mucosal Biology, King’s College London, National Health Service Guy’s and St. Thomas’ Trust, London, United Kingdom ABSTRACT The complement system, consisting of soluble and cell membrane–bound compo- weigh its effect on organ injury and nents of the innate immune system, has defined roles in the pathophysiology of renal rejection with dampening the antimi- allograft rejection. Notably, the unavoidable ischemia-reperfusion injury inherent to crobial functions of the complement sys- transplantation is mediated through the terminal complement activation products tem, such as opsonisation, cell lysis, and C5a and C5b-9. Furthermore, biologically active fragments C3a and C5a, produced recruitment of neutrophils and other in- during complement activation, can modulate both antigen presentation and T cell flammatory cells.7 It is our belief that priming, ultimately leading to allograft rejection. Earlier work identified renal tubule therapeutic strategies can be designed cell synthesis of C3, rather than hepatic synthesis of C3, as the primary source of C3 to specificallytargetthekeyinitiators driving these effects. Recent efforts have focused on identifying the local triggers of of complement activation at the relevant complement activation. Collectin-11, a soluble C-type lectin expressed in renal tis- location, such as complement-binding sue, has been implicated as an important trigger of complement activation in renal anti-HLA antibodies in the vascular tissue. In particular, collectin-11 has been shown to engage L-fucose at sites of compartment or the initiator(s) of local ischemic stress, activating the lectin complement pathway and directing the innate complement activation in the extravas- immune response to the distressed renal tubule. -

Supplementary Table 1: Adhesion Genes Data Set
Supplementary Table 1: Adhesion genes data set PROBE Entrez Gene ID Celera Gene ID Gene_Symbol Gene_Name 160832 1 hCG201364.3 A1BG alpha-1-B glycoprotein 223658 1 hCG201364.3 A1BG alpha-1-B glycoprotein 212988 102 hCG40040.3 ADAM10 ADAM metallopeptidase domain 10 133411 4185 hCG28232.2 ADAM11 ADAM metallopeptidase domain 11 110695 8038 hCG40937.4 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 195222 8038 hCG40937.4 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 165344 8751 hCG20021.3 ADAM15 ADAM metallopeptidase domain 15 (metargidin) 189065 6868 null ADAM17 ADAM metallopeptidase domain 17 (tumor necrosis factor, alpha, converting enzyme) 108119 8728 hCG15398.4 ADAM19 ADAM metallopeptidase domain 19 (meltrin beta) 117763 8748 hCG20675.3 ADAM20 ADAM metallopeptidase domain 20 126448 8747 hCG1785634.2 ADAM21 ADAM metallopeptidase domain 21 208981 8747 hCG1785634.2|hCG2042897 ADAM21 ADAM metallopeptidase domain 21 180903 53616 hCG17212.4 ADAM22 ADAM metallopeptidase domain 22 177272 8745 hCG1811623.1 ADAM23 ADAM metallopeptidase domain 23 102384 10863 hCG1818505.1 ADAM28 ADAM metallopeptidase domain 28 119968 11086 hCG1786734.2 ADAM29 ADAM metallopeptidase domain 29 205542 11085 hCG1997196.1 ADAM30 ADAM metallopeptidase domain 30 148417 80332 hCG39255.4 ADAM33 ADAM metallopeptidase domain 33 140492 8756 hCG1789002.2 ADAM7 ADAM metallopeptidase domain 7 122603 101 hCG1816947.1 ADAM8 ADAM metallopeptidase domain 8 183965 8754 hCG1996391 ADAM9 ADAM metallopeptidase domain 9 (meltrin gamma) 129974 27299 hCG15447.3 ADAMDEC1 ADAM-like, -

Complement Inactivation Strategy of Staphylococcus Aureus Using Decay-Accelerating Factor and the Response of Infected Hacat Cells
International Journal of Molecular Sciences Article Complement Inactivation Strategy of Staphylococcus aureus Using Decay-Accelerating Factor and the Response of Infected HaCaT Cells Kyoung Ok Jang 1, Youn Woo Lee 1, Hangeun Kim 2,* and Dae Kyun Chung 1,2,3,* 1 Graduate School of Biotechnology, Kyung Hee University, Yongin 17104, Korea; [email protected] (K.O.J.); [email protected] (Y.W.L.) 2 Research and Development Center, Skin Biotechnology Center Inc., Yongin 17104, Korea 3 Skin Biotechnology Center, Kyung Hee University, Suwon 16229, Korea * Correspondence: [email protected] (H.K.); [email protected] (D.K.C.); Tel.: +82-31-201-2487 (H.K.); +82-31-888-6170 (D.K.C.) Abstract: Staphylococcus aureus is a species of Gram-positive staphylococcus. It can cause sinusitis, respiratory infections, skin infections, and food poisoning. Recently, it was discovered that S. aureus infects epithelial cells, but the interaction between S. aureus and the host is not well known. In this study, we confirmed S. aureus to be internalized by HaCaT cells using the ESAT-6-like protein EsxB and amplified within the host over time by escaping host immunity. S. aureus increases the expression of decay-accelerating factor (CD55) on the surfaces of host cells, which inhibits the activation of the complement system. This mechanism makes it possible for S. aureus to survive in host cells. S. aureus, sufficiently amplified within the host, is released through the initiation of cell death. On the other hand, the infected host cells increase their surface expression of UL16 binding protein 1 to inform Citation: Jang, K.O.; Lee, Y.W.; Kim, immune cells that they are infected and try to be eliminated. -

Recognition of Microbial Glycans by Soluble Human Lectins
Available online at www.sciencedirect.com ScienceDirect Recognition of microbial glycans by soluble human lectins 3 1 1,2 Darryl A Wesener , Amanda Dugan and Laura L Kiessling Human innate immune lectins that recognize microbial glycans implicated in the regulation of microbial colonization and can conduct microbial surveillance and thereby help prevent in protection against infection. Seminal research on the infection. Structural analysis of soluble lectins has provided acute response to bacterial infection led to the identifica- invaluable insight into how these proteins recognize their tion of secreted factors that include C-reactive protein cognate carbohydrate ligands and how this recognition gives (CRP) and mannose-binding lectin (MBL) [1,3]. Both rise to biological function. In this opinion, we cover the CRP and MBL can recognize carbohydrate antigens on structural features of lectins that allow them to mediate the surface of pathogens, including Streptococcus pneumo- microbial recognition, highlighting examples from the collectin, niae and Staphylococcus aureus and then promote comple- Reg protein, galectin, pentraxin, ficolin and intelectin families. ment-mediated opsonization and cell killing [4]. Since These analyses reveal how some lectins (e.g., human intelectin- these initial observations, other lectins have been impli- 1) can recognize glycan epitopes that are remarkably diverse, cated in microbial recognition. Like MBL some of these yet still differentiate between mammalian and microbial proteins are C-type lectins, while others are members of glycans. We additionally discuss strategies to identify lectins the ficolin, pentraxin, galectin, or intelectin families. that recognize microbial glycans and highlight tools that Many of the lectins that function in microbial surveillance facilitate these discovery efforts. -
Elisa Kits and Monoclonal Antibodies for the Complement System
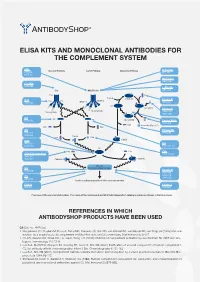
ELISA KITS AND MONOCLONAL ANTIBODIES FOR THE COMPLEMENT SYSTEM MBL ClassicalÊPathway LectinÊPathway AlternativeÊPathway H-Ficolin KITÊ029 RIGÊ334 HYBÊ131 M-Ficolin ABSÊ036 C1-INH HYBÊ288 L-Ficolin C1q MBL/Ficolin ABSÊ005 C1q B H2O C3Ê(H2O) FactorÊB C1s C1s C1r MASP-2 D ABSÊ002 MASP-1 HAVÊ005 C3 C3Ê(H O)ÊB 2 FactorÊD Carbohydrate Carbohydrate GAUÊ010 GAUÊ008 C2 C2 C4 C3Ê(H O)ÊBb HYBÊ050 2 Ba FactorÊBa/b HYBÊ008 C3a ProperdinÊ(FactorÊP) C2b C4a C3 Properdin HAVÊ004 HYBÊ0393 HYBÊ005 C4b2a C2a C4b C3bBb C3d HAVÊ003 C4 HYBÊ030 HYBÊ162-04 C3 C3a C3a C4b C3a/C3aÊ(desArg) HYBÊ162-02 GAUÊ013 GAUÊ017 C4b2a3b C3bBb3b C5Ê-ÊC9 C5 MembraneÊattackÊcomplex HYBÊ029 FactorÊH HYBÊ268 GAUÊ018 C9 GAUÊ020 ABSÊ004 LysisÊorÊphagocytosisÊofÊtheÊmicroorganism C5b-9 FactorÊI DIAÊ011 HYBÊ061 OverviewÊofÊtheÊcomplementÊsystem.ÊTheÊnameÊofÊtheÊcomponentÊandÊBioPortoÊDiagnostics'ÊcatalogÊnumberÊareÊshownÊinÊtheÊblueÊboxes. REFERENCES IN WHICH ANTIBODYSHOP PRODUCTS HAVE BEEN USED C2 Cat. no. HYB 050 Rooijakkers SH, Ruyken M, Roos A, Daha MR, Presanis JS, Sim RB, van Wamel WJ, van Kessel KP, van Strijp JA (2005) Immune evasion by a staphylococcal complement inhibitor that acts on C3 convertases. Nat Immunol 6:920-7. Oh KS, Kweon MH, Rhee KH, Ho Lee K, Sung HC (2003) Inhibition of complement activation by recombinant Sh-CRIT-ed1 ana- logues. Immunology 110:73-9. Laich A, Moffatt B, Wong KHN, Hickling TP, Koch C, Sim RB (2001) Purifi cation of second component of human complement, C2, by antibody affi nity chromatography. Intern J Bio-Chromatography 6:151-162. Laich A, Sim RB (2001) Complement C4bC2 complex formation: an investigation by surface plasmon resonance. Biochim Bio- phys Acta 1544:96-112. Stenbaek EI, Koch C, Barkholt V, Welinder KG (1986) Human complement component C2: production and characterization of polyclonal and monoclonal antibodies against C2. -

Table 1. Swine Proteins Identified As Differentially Expressed at 24Dpi in OURT 88/3 Infected Animals
Table 1. swine proteins identified as differentially expressed at 24dpi in OURT 88/3 infected animals. Gene name Protein ID Protein Name -Log p-value control vs A_24DPI Difference control Vs A_24DPI F8 K7GL28 Coagulation factor VIII 2.123919902 5.42533493 PPBP F1RUL6 C-X-C motif chemokine 3.219079808 4.493174871 SDPR I3LDR9 Caveolae associated protein 2 2.191007299 4.085711161 IGHG L8B0X5 IgG heavy chain 2.084611488 -4.282530149 LOC100517145 F1S3H9 Complement C3 (LOC100517145) 3.885740476 -4.364484406 GOLM1 F1S4I1 Golgi membrane protein 1 1.746130664 -4.767168681 FCN2 I3L5W3 Ficolin-2 2.937884686 -6.029483795 Table 2. swine proteins identified as differentially expressed at 7dpi in Benin ΔMGF infected animals. Gene name Protein ID Protein Name -Log p-value control vs B_7DPI Difference control Vs B_7DPI A0A075B7I5 Ig-like domain-containing protein 1.765578164 -3.480728149 ATP5A1 F1RPS8_PIG ATP synthase subunit alpha 2.270386995 3.270935059 LOC100627396 F1RX35_PIG Fibrinogen C-terminal domain-containing protein 2.211242648 3.967363358 LOC100514666;LOC102158263 F1RX36_PIG Fibrinogen alpha chain 2.337934993 3.758180618 FGB F1RX37_PIG Fibrinogen beta chain 2.411948004 4.03753376 PSMA8 F1SBA5_PIG Proteasome subunit alpha type 1.473601007 -3.815182686 ACAN F1SKR0_PIG Aggrecan core protein 1.974489764 -3.726634026 TFG F1SL01_PIG PB1 domain-containing protein 1.809215274 -3.131304741 LOC100154408 F1SSL6_PIG Proteasome subunit alpha type 1.701949053 -3.944885254 PSMA4 F2Z528_PIG Proteasome subunit alpha type 2.045768185 -4.502977371 PSMA5 F2Z5K2_PIG -

Journal of Proteomics 152 (2017) 131–137
Journal of Proteomics 152 (2017) 131–137 Contents lists available at ScienceDirect Journal of Proteomics journal homepage: www.elsevier.com/locate/jprot The Aotus nancymaae erythrocyte proteome and its importance for biomedical research D.A. Moreno-Pérez a,b, R. García-Valiente c, N. Ibarrola c,A.Murod,M.A.Patarroyoa,e,⁎ a Molecular Biology and Immunology Department, Fundación Instituto de Inmunología de Colombia (FIDIC), Carrera 50 No. 26–20, Bogotá, Colombia b PhD Programme in Biomedical and Biological Sciences, Universidad del Rosario, Carrera 24 No. 63C-69, Bogotá, Colombia c Unidad de Proteómica, Instituto de Biología Molecular y Celular del Cáncer -USAL-CSIC, ProteoRed ISCIII, Campus Unamuno, E-37007 Salamanca, Spain d Unidad de Investigación Enfermedades Infecciosas y Tropicales (e-INTRO), Instituto de Investigación Biomédica de Salamanca-Centro de Investigación de Enfermedades Tropicales de la Universidad de Salamanca (IBSAL-CIETUS), Facultad de Farmacia, Universidad de Salamanca, Campus Universitario Miguel de Unamuno s/n, E-37007 Salamanca, Spain e Basic Sciences Department, Universidad del Rosario, Carrera 24 No. 63C-69, Bogotá, Colombia article info abstract Article history: The Aotus nancymaae species has been of great importance in researching the biology and pathogenesis of malar- Received 9 August 2016 ia, particularly for studying Plasmodium molecules for including them in effective vaccines against such microor- Received in revised form 20 October 2016 ganism. In spite of the forgoing, there has been no report to date describing the biology of parasite target cells in Accepted 25 October 2016 primates or their biomedical importance. This study was thus designed to analyse A. nancymaae erythrocyte pro- Available online 29 October 2016 tein composition using MS data collected during a previous study aimed at characterising the Plasmodium vivax proteome and published in the pertinent literature. -

Ligation of Human CD46 with Purified Complement C3b Or F(Abo2 Of
J. Biochem. 132, 83-91 (2002) Ligation of Human CD46 with Purified Complement C3b or F(abO2 of Monoclonal Antibodies Enhances Isoform-Specific Interferon Gamma- Dependent Nitric Oxide Production in Macrophages1 Akiko Hirano,*2 Mitsue Kurita-TaniguchV Yuko Katayama,* Misako Matsumoto,1* Timocy C. Wong,*2 and Tsukasa Seyatw *Department of Microbiology, University of Washington. School of Medicine, Seattle, Washington, WA98195, USA; fDepartment of Immunology, Osaka Medical Center for Cancer and Cardiovascular Diseases, Higashinari-ku, Osaka 537-8511; and *CREST, JST (Japan Science and Technology Corporation), Kawaguchi, Tokyo 113-0022 Received April 8, 2002; accepted May 2, 2002 Downloaded from https://academic.oup.com/jb/article/132/1/83/891633 by guest on 27 September 2021 CD46, a complement regulatory protein widely expressed on human cells, serves as an entry receptor for measles virus (MV). We have previously shown that the expression of human CD46 in mouse macrophages restricts MV replication in these cells and enhances the production of nitric oxide (NO) in the presence of gamma interferon (IFN- 7). In this study, we show that crosslinking human CD46 expressed on the mouse mac- rophage-like cell line RAW264.7 with purified C3b multimer but not monomer enhances NO production. The enhanced production of NO in response to IFN-7 was observed again with C3b multimer but not monomer. The augmentation of NO production is human CD46-dependent with a CYT1>CYT2 profile. Thus, the reported MV-mediated NO production, irrespective of whether it is IFN-7-dependent or -independent, should be largely attributable to CD46 signaling but not to MV replication. -

Regenerative Therapy 6 (2017) 52E64
Regenerative Therapy 6 (2017) 52e64 Contents lists available at ScienceDirect Regenerative Therapy journal homepage: http://www.elsevier.com/locate/reth Original Article Phase I clinical study of liver regenerative therapy for cirrhosis by intrahepatic arterial infusion of freshly isolated autologous adipose tissue-derived stromal/stem (regenerative) cell Yoshio Sakai a, d, Masayuki Takamura b, c, Akihiro Seki c, Hajime Sunagozaka a, c, Takeshi Terashima a, Takuya Komura c, Masatoshi Yamato a, c, Masaki Miyazawa c, Kazunori Kawaguchi a, Alessandro Nasti c, Hatsune Mochida c, Soichiro Usui b, c, * Nobuhisa Otani c, Takahiro Ochiya e, Takashi Wada d, Masao Honda a, Shuichi Kaneko a, c, a Department of Gastroenterology, Kanazawa University Hospital, Japan b Department of Cardiology, Kanazawa University Hospital, Japan c System Biology, Kanazawa University, Japan d Department of Laboratory Medicine, Kanazawa University, Japan e Division of Molecular and Cellular Medicine, National Cancer Institute, Japan article info abstract Article history: Introduction: Adipose tissue stromal cells contain a substantial number of mesenchymal stem cells. As Received 14 September 2016 such, their application to regeneration of miscellaneous impaired organs has attracted much attention. Received in revised form Methods: We designed a clinical study to investigate freshly isolated autologous adipose tissue-derived 12 November 2016 stromal/stem (regenerative) cell (ADRC) therapy for liver cirrhosis and conducted treatment in four Accepted 8 December 2016 cirrhotic patients. ADRCs were isolated from autologous subcutaneous adipose tissue obtained by the liposuction method, followed with use of the Celution system adipose tissue dissociation device. The Keywords: primary endpoint is assessment of safety one month after treatment. We also characterized the obtained Adipose tissue-derived regenerative cells Stromal cells ADRCs. -

Ficolin-2 Triggers Antitumor and Anti-Pathogen Effects by Activating
Zhang XL. J Immunol Sci (2017); 1(1): 9-12 Journal of Immunological Sciences Mini Review Open Access Ficolin-2 triggers antitumor and anti-pathogen effects by activating antigen presenting cells and CD8+T cells Quanquan Ding, Min Liu, Xiao-Lian Zhang* State Key Laboratory of Virology and Medical Research Institute, Hubei Province Key Laboratory of Allergy and Immunology and Department of Immunology, Wuhan University School of Basic Medical Sciences, Wuhan 430071, P. R. China. Article Info ABSTRACT Article Notes Ficolins are important serum complement lectins with subunits consisting Received: October 25, 2017 of both collagen-like long thin stretches and fibrinogen-like globular domains Accepted: December 04, 2017 with lectin activity usually binding N-acetylglucosamine (GlcNAc). Like *Correspondence: collectins such as mannan-binding lectin, ficolins are secreted lectin-type Dr. Xiao-Lian Zhang, pattern recognition receptors, and activate the lectin pathway of complement State Key Laboratory of Virology and Medical Research activation1. Three human ficolins, namely, L-ficolin/P35 (FCN-2/ficolin-2), Institute, Hubei Province Key Laboratory of Allergy and M-ficolin (FCN-1/ficolin-1), and H-ficolin/Hakata antigen (FCN-3/ficolin-3), and Immunology and Department of Immunology, Wuhan two mouse ficolins (ficolins-A and -B) have been identified2,3. Mouse ficolin-A is University School of Basic Medical Sciences, Wuhan closely related to human ficolin-2. They are mainly synthesized in the liver and 430071, P. R. China, E-mail: [email protected] then secreted into blood circulation. © 2017 Zhang XL. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License. -

I M M U N O L O G Y Core Notes
II MM MM UU NN OO LL OO GG YY CCOORREE NNOOTTEESS MEDICAL IMMUNOLOGY 544 FALL 2011 Dr. George A. Gutman SCHOOL OF MEDICINE UNIVERSITY OF CALIFORNIA, IRVINE (Copyright) 2011 Regents of the University of California TABLE OF CONTENTS CHAPTER 1 INTRODUCTION...................................................................................... 3 CHAPTER 2 ANTIGEN/ANTIBODY INTERACTIONS ..............................................9 CHAPTER 3 ANTIBODY STRUCTURE I..................................................................17 CHAPTER 4 ANTIBODY STRUCTURE II.................................................................23 CHAPTER 5 COMPLEMENT...................................................................................... 33 CHAPTER 6 ANTIBODY GENETICS, ISOTYPES, ALLOTYPES, IDIOTYPES.....45 CHAPTER 7 CELLULAR BASIS OF ANTIBODY DIVERSITY: CLONAL SELECTION..................................................................53 CHAPTER 8 GENETIC BASIS OF ANTIBODY DIVERSITY...................................61 CHAPTER 9 IMMUNOGLOBULIN BIOSYNTHESIS ...............................................69 CHAPTER 10 BLOOD GROUPS: ABO AND Rh .........................................................77 CHAPTER 11 CELL-MEDIATED IMMUNITY AND MHC ........................................83 CHAPTER 12 CELL INTERACTIONS IN CELL MEDIATED IMMUNITY ..............91 CHAPTER 13 T-CELL/B-CELL COOPERATION IN HUMORAL IMMUNITY......105 CHAPTER 14 CELL SURFACE MARKERS OF T-CELLS, B-CELLS AND MACROPHAGES...............................................................111