Complement Inactivation Strategy of Staphylococcus Aureus Using Decay-Accelerating Factor and the Response of Infected Hacat Cells
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Complement Recognition Pathways in Renal Transplantation
BRIEF REVIEW www.jasn.org Complement Recognition Pathways in Renal Transplantation Christopher L. Nauser, Conrad A. Farrar, and Steven H. Sacks Medical Research Council Centre for Transplantation, Division of Transplantation Immunology and Mucosal Biology, King’s College London, National Health Service Guy’s and St. Thomas’ Trust, London, United Kingdom ABSTRACT The complement system, consisting of soluble and cell membrane–bound compo- weigh its effect on organ injury and nents of the innate immune system, has defined roles in the pathophysiology of renal rejection with dampening the antimi- allograft rejection. Notably, the unavoidable ischemia-reperfusion injury inherent to crobial functions of the complement sys- transplantation is mediated through the terminal complement activation products tem, such as opsonisation, cell lysis, and C5a and C5b-9. Furthermore, biologically active fragments C3a and C5a, produced recruitment of neutrophils and other in- during complement activation, can modulate both antigen presentation and T cell flammatory cells.7 It is our belief that priming, ultimately leading to allograft rejection. Earlier work identified renal tubule therapeutic strategies can be designed cell synthesis of C3, rather than hepatic synthesis of C3, as the primary source of C3 to specificallytargetthekeyinitiators driving these effects. Recent efforts have focused on identifying the local triggers of of complement activation at the relevant complement activation. Collectin-11, a soluble C-type lectin expressed in renal tis- location, such as complement-binding sue, has been implicated as an important trigger of complement activation in renal anti-HLA antibodies in the vascular tissue. In particular, collectin-11 has been shown to engage L-fucose at sites of compartment or the initiator(s) of local ischemic stress, activating the lectin complement pathway and directing the innate complement activation in the extravas- immune response to the distressed renal tubule. -

Activation Regulates Alternative Pathway Complement Necrotic
Properdin Binds to Late Apoptotic and Necrotic Cells Independently of C3b and Regulates Alternative Pathway Complement Activation This information is current as of September 27, 2021. Wei Xu, Stefan P. Berger, Leendert A. Trouw, Hetty C. de Boer, Nicole Schlagwein, Chantal Mutsaers, Mohamed R. Daha and Cees van Kooten J Immunol 2008; 180:7613-7621; ; doi: 10.4049/jimmunol.180.11.7613 Downloaded from http://www.jimmunol.org/content/180/11/7613 References This article cites 56 articles, 27 of which you can access for free at: http://www.jimmunol.org/ http://www.jimmunol.org/content/180/11/7613.full#ref-list-1 Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists by guest on September 27, 2021 • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2008 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Properdin Binds to Late Apoptotic and Necrotic Cells Independently of C3b and Regulates Alternative Pathway Complement Activation1,2 Wei Xu,* Stefan P. Berger,* Leendert A. -

Supplementary Table 1: Adhesion Genes Data Set
Supplementary Table 1: Adhesion genes data set PROBE Entrez Gene ID Celera Gene ID Gene_Symbol Gene_Name 160832 1 hCG201364.3 A1BG alpha-1-B glycoprotein 223658 1 hCG201364.3 A1BG alpha-1-B glycoprotein 212988 102 hCG40040.3 ADAM10 ADAM metallopeptidase domain 10 133411 4185 hCG28232.2 ADAM11 ADAM metallopeptidase domain 11 110695 8038 hCG40937.4 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 195222 8038 hCG40937.4 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 165344 8751 hCG20021.3 ADAM15 ADAM metallopeptidase domain 15 (metargidin) 189065 6868 null ADAM17 ADAM metallopeptidase domain 17 (tumor necrosis factor, alpha, converting enzyme) 108119 8728 hCG15398.4 ADAM19 ADAM metallopeptidase domain 19 (meltrin beta) 117763 8748 hCG20675.3 ADAM20 ADAM metallopeptidase domain 20 126448 8747 hCG1785634.2 ADAM21 ADAM metallopeptidase domain 21 208981 8747 hCG1785634.2|hCG2042897 ADAM21 ADAM metallopeptidase domain 21 180903 53616 hCG17212.4 ADAM22 ADAM metallopeptidase domain 22 177272 8745 hCG1811623.1 ADAM23 ADAM metallopeptidase domain 23 102384 10863 hCG1818505.1 ADAM28 ADAM metallopeptidase domain 28 119968 11086 hCG1786734.2 ADAM29 ADAM metallopeptidase domain 29 205542 11085 hCG1997196.1 ADAM30 ADAM metallopeptidase domain 30 148417 80332 hCG39255.4 ADAM33 ADAM metallopeptidase domain 33 140492 8756 hCG1789002.2 ADAM7 ADAM metallopeptidase domain 7 122603 101 hCG1816947.1 ADAM8 ADAM metallopeptidase domain 8 183965 8754 hCG1996391 ADAM9 ADAM metallopeptidase domain 9 (meltrin gamma) 129974 27299 hCG15447.3 ADAMDEC1 ADAM-like, -

Recognition of Microbial Glycans by Soluble Human Lectins
Available online at www.sciencedirect.com ScienceDirect Recognition of microbial glycans by soluble human lectins 3 1 1,2 Darryl A Wesener , Amanda Dugan and Laura L Kiessling Human innate immune lectins that recognize microbial glycans implicated in the regulation of microbial colonization and can conduct microbial surveillance and thereby help prevent in protection against infection. Seminal research on the infection. Structural analysis of soluble lectins has provided acute response to bacterial infection led to the identifica- invaluable insight into how these proteins recognize their tion of secreted factors that include C-reactive protein cognate carbohydrate ligands and how this recognition gives (CRP) and mannose-binding lectin (MBL) [1,3]. Both rise to biological function. In this opinion, we cover the CRP and MBL can recognize carbohydrate antigens on structural features of lectins that allow them to mediate the surface of pathogens, including Streptococcus pneumo- microbial recognition, highlighting examples from the collectin, niae and Staphylococcus aureus and then promote comple- Reg protein, galectin, pentraxin, ficolin and intelectin families. ment-mediated opsonization and cell killing [4]. Since These analyses reveal how some lectins (e.g., human intelectin- these initial observations, other lectins have been impli- 1) can recognize glycan epitopes that are remarkably diverse, cated in microbial recognition. Like MBL some of these yet still differentiate between mammalian and microbial proteins are C-type lectins, while others are members of glycans. We additionally discuss strategies to identify lectins the ficolin, pentraxin, galectin, or intelectin families. that recognize microbial glycans and highlight tools that Many of the lectins that function in microbial surveillance facilitate these discovery efforts. -
Elisa Kits and Monoclonal Antibodies for the Complement System
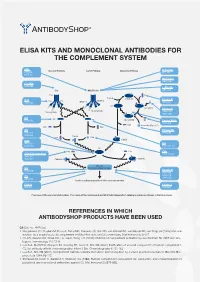
ELISA KITS AND MONOCLONAL ANTIBODIES FOR THE COMPLEMENT SYSTEM MBL ClassicalÊPathway LectinÊPathway AlternativeÊPathway H-Ficolin KITÊ029 RIGÊ334 HYBÊ131 M-Ficolin ABSÊ036 C1-INH HYBÊ288 L-Ficolin C1q MBL/Ficolin ABSÊ005 C1q B H2O C3Ê(H2O) FactorÊB C1s C1s C1r MASP-2 D ABSÊ002 MASP-1 HAVÊ005 C3 C3Ê(H O)ÊB 2 FactorÊD Carbohydrate Carbohydrate GAUÊ010 GAUÊ008 C2 C2 C4 C3Ê(H O)ÊBb HYBÊ050 2 Ba FactorÊBa/b HYBÊ008 C3a ProperdinÊ(FactorÊP) C2b C4a C3 Properdin HAVÊ004 HYBÊ0393 HYBÊ005 C4b2a C2a C4b C3bBb C3d HAVÊ003 C4 HYBÊ030 HYBÊ162-04 C3 C3a C3a C4b C3a/C3aÊ(desArg) HYBÊ162-02 GAUÊ013 GAUÊ017 C4b2a3b C3bBb3b C5Ê-ÊC9 C5 MembraneÊattackÊcomplex HYBÊ029 FactorÊH HYBÊ268 GAUÊ018 C9 GAUÊ020 ABSÊ004 LysisÊorÊphagocytosisÊofÊtheÊmicroorganism C5b-9 FactorÊI DIAÊ011 HYBÊ061 OverviewÊofÊtheÊcomplementÊsystem.ÊTheÊnameÊofÊtheÊcomponentÊandÊBioPortoÊDiagnostics'ÊcatalogÊnumberÊareÊshownÊinÊtheÊblueÊboxes. REFERENCES IN WHICH ANTIBODYSHOP PRODUCTS HAVE BEEN USED C2 Cat. no. HYB 050 Rooijakkers SH, Ruyken M, Roos A, Daha MR, Presanis JS, Sim RB, van Wamel WJ, van Kessel KP, van Strijp JA (2005) Immune evasion by a staphylococcal complement inhibitor that acts on C3 convertases. Nat Immunol 6:920-7. Oh KS, Kweon MH, Rhee KH, Ho Lee K, Sung HC (2003) Inhibition of complement activation by recombinant Sh-CRIT-ed1 ana- logues. Immunology 110:73-9. Laich A, Moffatt B, Wong KHN, Hickling TP, Koch C, Sim RB (2001) Purifi cation of second component of human complement, C2, by antibody affi nity chromatography. Intern J Bio-Chromatography 6:151-162. Laich A, Sim RB (2001) Complement C4bC2 complex formation: an investigation by surface plasmon resonance. Biochim Bio- phys Acta 1544:96-112. Stenbaek EI, Koch C, Barkholt V, Welinder KG (1986) Human complement component C2: production and characterization of polyclonal and monoclonal antibodies against C2. -

Table 1. Swine Proteins Identified As Differentially Expressed at 24Dpi in OURT 88/3 Infected Animals
Table 1. swine proteins identified as differentially expressed at 24dpi in OURT 88/3 infected animals. Gene name Protein ID Protein Name -Log p-value control vs A_24DPI Difference control Vs A_24DPI F8 K7GL28 Coagulation factor VIII 2.123919902 5.42533493 PPBP F1RUL6 C-X-C motif chemokine 3.219079808 4.493174871 SDPR I3LDR9 Caveolae associated protein 2 2.191007299 4.085711161 IGHG L8B0X5 IgG heavy chain 2.084611488 -4.282530149 LOC100517145 F1S3H9 Complement C3 (LOC100517145) 3.885740476 -4.364484406 GOLM1 F1S4I1 Golgi membrane protein 1 1.746130664 -4.767168681 FCN2 I3L5W3 Ficolin-2 2.937884686 -6.029483795 Table 2. swine proteins identified as differentially expressed at 7dpi in Benin ΔMGF infected animals. Gene name Protein ID Protein Name -Log p-value control vs B_7DPI Difference control Vs B_7DPI A0A075B7I5 Ig-like domain-containing protein 1.765578164 -3.480728149 ATP5A1 F1RPS8_PIG ATP synthase subunit alpha 2.270386995 3.270935059 LOC100627396 F1RX35_PIG Fibrinogen C-terminal domain-containing protein 2.211242648 3.967363358 LOC100514666;LOC102158263 F1RX36_PIG Fibrinogen alpha chain 2.337934993 3.758180618 FGB F1RX37_PIG Fibrinogen beta chain 2.411948004 4.03753376 PSMA8 F1SBA5_PIG Proteasome subunit alpha type 1.473601007 -3.815182686 ACAN F1SKR0_PIG Aggrecan core protein 1.974489764 -3.726634026 TFG F1SL01_PIG PB1 domain-containing protein 1.809215274 -3.131304741 LOC100154408 F1SSL6_PIG Proteasome subunit alpha type 1.701949053 -3.944885254 PSMA4 F2Z528_PIG Proteasome subunit alpha type 2.045768185 -4.502977371 PSMA5 F2Z5K2_PIG -

Regenerative Therapy 6 (2017) 52E64
Regenerative Therapy 6 (2017) 52e64 Contents lists available at ScienceDirect Regenerative Therapy journal homepage: http://www.elsevier.com/locate/reth Original Article Phase I clinical study of liver regenerative therapy for cirrhosis by intrahepatic arterial infusion of freshly isolated autologous adipose tissue-derived stromal/stem (regenerative) cell Yoshio Sakai a, d, Masayuki Takamura b, c, Akihiro Seki c, Hajime Sunagozaka a, c, Takeshi Terashima a, Takuya Komura c, Masatoshi Yamato a, c, Masaki Miyazawa c, Kazunori Kawaguchi a, Alessandro Nasti c, Hatsune Mochida c, Soichiro Usui b, c, * Nobuhisa Otani c, Takahiro Ochiya e, Takashi Wada d, Masao Honda a, Shuichi Kaneko a, c, a Department of Gastroenterology, Kanazawa University Hospital, Japan b Department of Cardiology, Kanazawa University Hospital, Japan c System Biology, Kanazawa University, Japan d Department of Laboratory Medicine, Kanazawa University, Japan e Division of Molecular and Cellular Medicine, National Cancer Institute, Japan article info abstract Article history: Introduction: Adipose tissue stromal cells contain a substantial number of mesenchymal stem cells. As Received 14 September 2016 such, their application to regeneration of miscellaneous impaired organs has attracted much attention. Received in revised form Methods: We designed a clinical study to investigate freshly isolated autologous adipose tissue-derived 12 November 2016 stromal/stem (regenerative) cell (ADRC) therapy for liver cirrhosis and conducted treatment in four Accepted 8 December 2016 cirrhotic patients. ADRCs were isolated from autologous subcutaneous adipose tissue obtained by the liposuction method, followed with use of the Celution system adipose tissue dissociation device. The Keywords: primary endpoint is assessment of safety one month after treatment. We also characterized the obtained Adipose tissue-derived regenerative cells Stromal cells ADRCs. -

Ficolin-2 Triggers Antitumor and Anti-Pathogen Effects by Activating
Zhang XL. J Immunol Sci (2017); 1(1): 9-12 Journal of Immunological Sciences Mini Review Open Access Ficolin-2 triggers antitumor and anti-pathogen effects by activating antigen presenting cells and CD8+T cells Quanquan Ding, Min Liu, Xiao-Lian Zhang* State Key Laboratory of Virology and Medical Research Institute, Hubei Province Key Laboratory of Allergy and Immunology and Department of Immunology, Wuhan University School of Basic Medical Sciences, Wuhan 430071, P. R. China. Article Info ABSTRACT Article Notes Ficolins are important serum complement lectins with subunits consisting Received: October 25, 2017 of both collagen-like long thin stretches and fibrinogen-like globular domains Accepted: December 04, 2017 with lectin activity usually binding N-acetylglucosamine (GlcNAc). Like *Correspondence: collectins such as mannan-binding lectin, ficolins are secreted lectin-type Dr. Xiao-Lian Zhang, pattern recognition receptors, and activate the lectin pathway of complement State Key Laboratory of Virology and Medical Research activation1. Three human ficolins, namely, L-ficolin/P35 (FCN-2/ficolin-2), Institute, Hubei Province Key Laboratory of Allergy and M-ficolin (FCN-1/ficolin-1), and H-ficolin/Hakata antigen (FCN-3/ficolin-3), and Immunology and Department of Immunology, Wuhan two mouse ficolins (ficolins-A and -B) have been identified2,3. Mouse ficolin-A is University School of Basic Medical Sciences, Wuhan closely related to human ficolin-2. They are mainly synthesized in the liver and 430071, P. R. China, E-mail: [email protected] then secreted into blood circulation. © 2017 Zhang XL. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License. -

Ficolin B in Diabetic Kidney Disease in a Mouse Model of Type 1 Diabetes
Hindawi Publishing Corporation Mediators of Inflammation Volume 2015, Article ID 653260, 6 pages http://dx.doi.org/10.1155/2015/653260 Research Article Ficolin B in Diabetic Kidney Disease in a Mouse Model of Type 1 Diabetes Charlotte Berg Holt,1,2 Jakob Appel Østergaard,1,3 Esben Axelgaard,2 Gitte Krogh Nielsen,2 Yuichi Endo,4 Steffen Thiel,2 and Troels Krarup Hansen1 1 Department of Endocrinology and Internal Medicine, Aarhus University Hospital, 8000 Aarhus, Denmark 2Department of Biomedicine, Aarhus University, 8000 Aarhus, Denmark 3The Danish Diabetes Academy, Sdr. Boulevard 29, 5000 Odense, Denmark 4Department of Immunology, Fukushima Medical University School of Medicine, Fukushima 960-1295, Japan Correspondence should be addressed to Troels Krarup Hansen; [email protected] Received 5 August 2014; Accepted 12 October 2014 Academic Editor: Peter Huszthy Copyright © 2015 Charlotte Berg Holt et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Background. The innate immune system may have adverse effects in diabetes and cardiovascular disease. The complement system seems to play a key role through erroneous complement activation via hyperglycaemia-induced neoepitopes. Recently mannan- binding lectin (MBL) was shown to worsen diabetic kidney changes. We hypothesize that mouse ficolin B exerts detrimental effects in the diabetic kidney as seen for MBL. Methods. We induced diabetes with streptozotocin in female wild-type mice and ficolin B knockout mice and included two similar nondiabetic groups. Renal hypertrophy and excretion of urinary albumin and creatinine were quantified to assess diabetic kidney damage. -

The Human Soluble Lectin L-Ficolin Neutralizes Entry of Hepatitis C
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Repository@Nottingham 1 Recombinant Human L-Ficolin Directly Neutralizes Hepatitis C Virus Entry 2 3 Running title: L-ficolin neutralizes HCV entry 4 1,2 1† 3 1 5 Mohamed R. Hamed , Richard J. P. Brown , Carsten Zothner , Richard A. Urbanowicz , 6 Christopher P. Mason1, Anders Krarup4, C. Patrick McClure1, William L. Irving1, 7 Jonathan K. Ball1, Mark Harris3, Timothy P. Hickling1‡ & Alexander W. Tarr1# 8 9 1 School of Life Sciences, and Nottingham Digestive Diseases Biomedical Research Unit, 10 University of Nottingham, UK. 11 2 Medical Microbiology and Immunology Department, Faculty of Medicine, Mansoura 12 University, Egypt 13 3 School of Molecular and Cellular Biology, Faculty of Biological Sciences, University of 14 Leeds, UK 15 4 Biochemistry Department, University of Oxford, UK 16 17 #Corresponding author: Dr Alexander W. Tarr, Virus Research Group, School of Life 18 Sciences, Queen’s Medical Centre, University of Nottingham, Nottingham NG7 2UH. Tel: 19 01158230746; Fax: 01158230759; email: [email protected] 20 †Current address: TWINCORE Zentrum für Experimentelle und Klinische 21 Infektionsforschung GmbH,Feodor-Lynen-Str. 7,30625 Hannover, Germany 22 Current address: Crucell, Archimedesweg 4-6, 2333 CN Leiden, Netherlands 23 ‡Current address: Pfizer, Pharmacokinetics, Dynamics and Metabolism, 1 Burtt Road, 24 Andover, MA 01810, USA. 25 26 Abstract word count: 155 27 Main text word count: 3747 28 Key words: 29 hepatitis c virus; ficolin; virus entry; neutralization; glycosylation 30 Abstract 31 32 L-ficolin is a liver-expressed soluble pattern recognition molecule that contributes to innate 33 immune defense against microorganisms. -

The Evolution of the Immune System: Conservation and Diversification
Title The Evolution of the Immune System Conservation and Diversification Page left intentionally blank The Evolution of the Immune System Conservation and Diversification Davide Malagoli Department of Life Sciences Biology Building, University of Modena and Reggio Emilia, Modena, Italy AMSTERDAM • BOSTON • HEIDELBERG • LONDON NEW YORK • OXFORD • PARIS • SAN DIEGO SAN FRANCISCO • SINGAPORE • SYDNEY • TOKYO Academic Press is an imprint of Elsevier Academic Press is an imprint of Elsevier 125 London Wall, London EC2Y 5AS, United Kingdom 525 B Street, Suite 1800, San Diego, CA 92101-4495, United States 50 Hampshire Street, 5th Floor, Cambridge, MA 02139, United States The Boulevard, Langford Lane, Kidlington, Oxford OX5 1GB, UK Copyright © 2016 Elsevier Inc. All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or any information storage and retrieval system, without permission in writing from the publisher. Details on how to seek per- mission, further information about the Publisher’s permissions policies and our arrangements with organizations such as the Copyright Clearance Center and the Copyright Licensing Agency, can be found at our website: www.elsevier.com/permissions. This book and the individual contributions contained in it are protected under copyright by the Publisher (other than as may be noted herein). Notices Knowledge and best practice in this field are constantly changing. As new research and experience broaden our understanding, changes in research methods, professional practices, or medical treatment may become necessary. Practitioners and researchers must always rely on their own experience and knowledge in evaluating and using any information, methods, compounds, or experiments described herein. -

Intracellular Complement Activation—An Alarm Raising Mechanism? T ⁎ ⁎⁎ M.P
Seminars in Immunology 38 (2018) 54–62 Contents lists available at ScienceDirect Seminars in Immunology journal homepage: www.elsevier.com/locate/ysmim Review Intracellular complement activation—An alarm raising mechanism? T ⁎ ⁎⁎ M.P. Reichhardta, , S. Merib,c, a Sir William Dunn School of Pathology, University of Oxford, Oxford, United Kingdom b Department of Bacteriology and Immunology, Haartman Institute, Immunobiology Research Program, University of Helsinki, Helsinki, Finland c Helsinki University Central Hospital Laboratory (HUSLAB), Helsinki, Finland ARTICLE INFO ABSTRACT Keywords: It has become increasingly apparent that the complement system, being an ancient defense mechanism, is not Intracellular complement operative only in the extracellular milieu but also intracellularly. In addition to the known synthetic machinery C3 in the liver and by macrophages, many other cell types, including lymphocytes, adipocytes and epithelial cells C5 produce selected complement components. Activation of e.g. C3 and C5 inside cells may have multiple effects C3a ranging from direct antimicrobial defense to cell differentiation and possible influence on metabolism. C5a Intracellular activation of C3 and C5 in T cells is involved in the maintenance of immunological tolerance and Inflammasome ff Complement promotes di erentiation of T helper cells into Th1-type cells that activate cell-mediated immune responses. C5aR1 Adipocytes are unique in producing many complement sensor proteins (like C1q) and Factor D (adipsin), the key C5aR2 enzyme in promoting alternative pathway amplification. The effects of complement activation products are Metabolism mediated by intracellular and cell membrane receptors, like C3aR, C5aR1, C5aR2 and the complement regulator Cellular homeostasis MCP/CD46, often jointly with other receptors like the T cell receptor, Toll-like receptors and those of the in- Complement signaling flammasomes.