Amyotrophic Lateral Sclerosis and the Innate Immune System: Protocol for Establishing a Biobank and Statistical Analysis Plan
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pentraxin-2 Suppresses C-Jun/AP-1 Signaling to Inhibit Progressive Fibrotic Disease
Pentraxin-2 suppresses c-Jun/AP-1 signaling to inhibit progressive fibrotic disease Naoki Nakagawa, … , Sina A. Gharib, Jeremy S. Duffield JCI Insight. 2016;1(20):e87446. https://doi.org/10.1172/jci.insight.87446. Research Article Immunology Inflammation Pentraxin-2 (PTX-2), also known as serum amyloid P component (SAP/APCS), is a constitutive, antiinflammatory, innate immune plasma protein whose circulating level is decreased in chronic human fibrotic diseases. Here we show that recombinant human PTX-2 (rhPTX-2) retards progression of chronic kidney disease in Col4a3 mutant mice with Alport syndrome, reducing blood markers of kidney failure, enhancing lifespan by 20%, and improving histological signs of disease. Exogenously delivered rhPTX-2 was detected in macrophages but also in tubular epithelial cells, where it counteracted macrophage activation and was cytoprotective for the epithelium. Computational analysis of genes regulated by rhPTX-2 identified the transcriptional regulator c-Jun along with its activator protein–1 (AP-1) binding partners as a central target for the function of rhPTX-2. Accordingly, PTX-2 attenuates c-Jun and AP-1 activity, and reduces expression of AP-1–dependent inflammatory genes in both monocytes and epithelium. Our studies therefore identify rhPTX-2 as a potential therapy for chronic fibrotic disease of the kidney and an important inhibitor of pathological c-Jun signaling in this setting. Find the latest version: https://jci.me/87446/pdf RESEARCH ARTICLE Pentraxin-2 suppresses c-Jun/AP-1 signaling to inhibit progressive fibrotic disease Naoki Nakagawa,1,2,3 Luke Barron,4 Ivan G. Gomez,1,2,4 Bryce G. -

Complement Recognition Pathways in Renal Transplantation
BRIEF REVIEW www.jasn.org Complement Recognition Pathways in Renal Transplantation Christopher L. Nauser, Conrad A. Farrar, and Steven H. Sacks Medical Research Council Centre for Transplantation, Division of Transplantation Immunology and Mucosal Biology, King’s College London, National Health Service Guy’s and St. Thomas’ Trust, London, United Kingdom ABSTRACT The complement system, consisting of soluble and cell membrane–bound compo- weigh its effect on organ injury and nents of the innate immune system, has defined roles in the pathophysiology of renal rejection with dampening the antimi- allograft rejection. Notably, the unavoidable ischemia-reperfusion injury inherent to crobial functions of the complement sys- transplantation is mediated through the terminal complement activation products tem, such as opsonisation, cell lysis, and C5a and C5b-9. Furthermore, biologically active fragments C3a and C5a, produced recruitment of neutrophils and other in- during complement activation, can modulate both antigen presentation and T cell flammatory cells.7 It is our belief that priming, ultimately leading to allograft rejection. Earlier work identified renal tubule therapeutic strategies can be designed cell synthesis of C3, rather than hepatic synthesis of C3, as the primary source of C3 to specificallytargetthekeyinitiators driving these effects. Recent efforts have focused on identifying the local triggers of of complement activation at the relevant complement activation. Collectin-11, a soluble C-type lectin expressed in renal tis- location, such as complement-binding sue, has been implicated as an important trigger of complement activation in renal anti-HLA antibodies in the vascular tissue. In particular, collectin-11 has been shown to engage L-fucose at sites of compartment or the initiator(s) of local ischemic stress, activating the lectin complement pathway and directing the innate complement activation in the extravas- immune response to the distressed renal tubule. -

Pentraxin-3 to Better Delineate Necrotizing Soft Tissue Infection: Not Really! Patrick M
Honore and Spapen Critical Care (2016) 20:173 DOI 10.1186/s13054-016-1319-0 LETTER Open Access Pentraxin-3 to better delineate necrotizing soft tissue infection: not really! Patrick M. Honore* and Herbert D. Spapen See related research by Hansen et al., http://ccforum.biomedcentral.com/articles/10.1186/s13054-016-1210-z Necrotizing soft tissue infection (NSTI) is a devastating assessment and persistently elevated levels during evolv- condition with high morbidity and a dismal prognosis. ing sepsis may contribute to tissue damage and predict Timely and adequate surgery and early aggressive treat- poor patient outcome [2]. Second, when looking closely ment of associated sepsis are imperative to improve at the study results, PTX3 levels appeared to be fairly in survival [1–3]. Pentraxin-3 (PTX3) is a glycoprotein line with PCT concentrations for assessing NSTI- released by endothelial and inflammatory cells upon associated morbidity and mortality. PCT also outper- stimulation by cytokines and endotoxins. In contrast with forms CRP in predicting severity of infection and organ C-reactive protein (CRP) which is produced in the liver in dysfunction in sepsis [4] but is more readily available response to systemic inflammation, PTX3 is thought to than PTX3. Finally, many patients with NSTI develop better reflect local vascular inflammation and bacterial acute kidney injury, necessitating renal replacement load [2]. PTX3 assessment might thus be a more appropri- therapy (RRT) (25 % of the patients studied by Hansen ate marker of severity and prognosis of NSTI. This was et al.!). PTX3 has a molecular weight of approximately corroborated by Hansen et al. -

Activation Regulates Alternative Pathway Complement Necrotic
Properdin Binds to Late Apoptotic and Necrotic Cells Independently of C3b and Regulates Alternative Pathway Complement Activation This information is current as of September 27, 2021. Wei Xu, Stefan P. Berger, Leendert A. Trouw, Hetty C. de Boer, Nicole Schlagwein, Chantal Mutsaers, Mohamed R. Daha and Cees van Kooten J Immunol 2008; 180:7613-7621; ; doi: 10.4049/jimmunol.180.11.7613 Downloaded from http://www.jimmunol.org/content/180/11/7613 References This article cites 56 articles, 27 of which you can access for free at: http://www.jimmunol.org/ http://www.jimmunol.org/content/180/11/7613.full#ref-list-1 Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists by guest on September 27, 2021 • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2008 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Properdin Binds to Late Apoptotic and Necrotic Cells Independently of C3b and Regulates Alternative Pathway Complement Activation1,2 Wei Xu,* Stefan P. Berger,* Leendert A. -

Supplementary Table 1: Adhesion Genes Data Set
Supplementary Table 1: Adhesion genes data set PROBE Entrez Gene ID Celera Gene ID Gene_Symbol Gene_Name 160832 1 hCG201364.3 A1BG alpha-1-B glycoprotein 223658 1 hCG201364.3 A1BG alpha-1-B glycoprotein 212988 102 hCG40040.3 ADAM10 ADAM metallopeptidase domain 10 133411 4185 hCG28232.2 ADAM11 ADAM metallopeptidase domain 11 110695 8038 hCG40937.4 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 195222 8038 hCG40937.4 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 165344 8751 hCG20021.3 ADAM15 ADAM metallopeptidase domain 15 (metargidin) 189065 6868 null ADAM17 ADAM metallopeptidase domain 17 (tumor necrosis factor, alpha, converting enzyme) 108119 8728 hCG15398.4 ADAM19 ADAM metallopeptidase domain 19 (meltrin beta) 117763 8748 hCG20675.3 ADAM20 ADAM metallopeptidase domain 20 126448 8747 hCG1785634.2 ADAM21 ADAM metallopeptidase domain 21 208981 8747 hCG1785634.2|hCG2042897 ADAM21 ADAM metallopeptidase domain 21 180903 53616 hCG17212.4 ADAM22 ADAM metallopeptidase domain 22 177272 8745 hCG1811623.1 ADAM23 ADAM metallopeptidase domain 23 102384 10863 hCG1818505.1 ADAM28 ADAM metallopeptidase domain 28 119968 11086 hCG1786734.2 ADAM29 ADAM metallopeptidase domain 29 205542 11085 hCG1997196.1 ADAM30 ADAM metallopeptidase domain 30 148417 80332 hCG39255.4 ADAM33 ADAM metallopeptidase domain 33 140492 8756 hCG1789002.2 ADAM7 ADAM metallopeptidase domain 7 122603 101 hCG1816947.1 ADAM8 ADAM metallopeptidase domain 8 183965 8754 hCG1996391 ADAM9 ADAM metallopeptidase domain 9 (meltrin gamma) 129974 27299 hCG15447.3 ADAMDEC1 ADAM-like, -

Complement Inactivation Strategy of Staphylococcus Aureus Using Decay-Accelerating Factor and the Response of Infected Hacat Cells
International Journal of Molecular Sciences Article Complement Inactivation Strategy of Staphylococcus aureus Using Decay-Accelerating Factor and the Response of Infected HaCaT Cells Kyoung Ok Jang 1, Youn Woo Lee 1, Hangeun Kim 2,* and Dae Kyun Chung 1,2,3,* 1 Graduate School of Biotechnology, Kyung Hee University, Yongin 17104, Korea; [email protected] (K.O.J.); [email protected] (Y.W.L.) 2 Research and Development Center, Skin Biotechnology Center Inc., Yongin 17104, Korea 3 Skin Biotechnology Center, Kyung Hee University, Suwon 16229, Korea * Correspondence: [email protected] (H.K.); [email protected] (D.K.C.); Tel.: +82-31-201-2487 (H.K.); +82-31-888-6170 (D.K.C.) Abstract: Staphylococcus aureus is a species of Gram-positive staphylococcus. It can cause sinusitis, respiratory infections, skin infections, and food poisoning. Recently, it was discovered that S. aureus infects epithelial cells, but the interaction between S. aureus and the host is not well known. In this study, we confirmed S. aureus to be internalized by HaCaT cells using the ESAT-6-like protein EsxB and amplified within the host over time by escaping host immunity. S. aureus increases the expression of decay-accelerating factor (CD55) on the surfaces of host cells, which inhibits the activation of the complement system. This mechanism makes it possible for S. aureus to survive in host cells. S. aureus, sufficiently amplified within the host, is released through the initiation of cell death. On the other hand, the infected host cells increase their surface expression of UL16 binding protein 1 to inform Citation: Jang, K.O.; Lee, Y.W.; Kim, immune cells that they are infected and try to be eliminated. -

Recognition of Microbial Glycans by Soluble Human Lectins
Available online at www.sciencedirect.com ScienceDirect Recognition of microbial glycans by soluble human lectins 3 1 1,2 Darryl A Wesener , Amanda Dugan and Laura L Kiessling Human innate immune lectins that recognize microbial glycans implicated in the regulation of microbial colonization and can conduct microbial surveillance and thereby help prevent in protection against infection. Seminal research on the infection. Structural analysis of soluble lectins has provided acute response to bacterial infection led to the identifica- invaluable insight into how these proteins recognize their tion of secreted factors that include C-reactive protein cognate carbohydrate ligands and how this recognition gives (CRP) and mannose-binding lectin (MBL) [1,3]. Both rise to biological function. In this opinion, we cover the CRP and MBL can recognize carbohydrate antigens on structural features of lectins that allow them to mediate the surface of pathogens, including Streptococcus pneumo- microbial recognition, highlighting examples from the collectin, niae and Staphylococcus aureus and then promote comple- Reg protein, galectin, pentraxin, ficolin and intelectin families. ment-mediated opsonization and cell killing [4]. Since These analyses reveal how some lectins (e.g., human intelectin- these initial observations, other lectins have been impli- 1) can recognize glycan epitopes that are remarkably diverse, cated in microbial recognition. Like MBL some of these yet still differentiate between mammalian and microbial proteins are C-type lectins, while others are members of glycans. We additionally discuss strategies to identify lectins the ficolin, pentraxin, galectin, or intelectin families. that recognize microbial glycans and highlight tools that Many of the lectins that function in microbial surveillance facilitate these discovery efforts. -

Human Lectins, Their Carbohydrate Affinities and Where to Find Them
biomolecules Review Human Lectins, Their Carbohydrate Affinities and Where to Review HumanFind Them Lectins, Their Carbohydrate Affinities and Where to FindCláudia ThemD. Raposo 1,*, André B. Canelas 2 and M. Teresa Barros 1 1, 2 1 Cláudia D. Raposo * , Andr1 é LAQVB. Canelas‐Requimte,and Department M. Teresa of Chemistry, Barros NOVA School of Science and Technology, Universidade NOVA de Lisboa, 2829‐516 Caparica, Portugal; [email protected] 12 GlanbiaLAQV-Requimte,‐AgriChemWhey, Department Lisheen of Chemistry, Mine, Killoran, NOVA Moyne, School E41 of ScienceR622 Co. and Tipperary, Technology, Ireland; canelas‐ [email protected] NOVA de Lisboa, 2829-516 Caparica, Portugal; [email protected] 2* Correspondence:Glanbia-AgriChemWhey, [email protected]; Lisheen Mine, Tel.: Killoran, +351‐212948550 Moyne, E41 R622 Tipperary, Ireland; [email protected] * Correspondence: [email protected]; Tel.: +351-212948550 Abstract: Lectins are a class of proteins responsible for several biological roles such as cell‐cell in‐ Abstract:teractions,Lectins signaling are pathways, a class of and proteins several responsible innate immune for several responses biological against roles pathogens. such as Since cell-cell lec‐ interactions,tins are able signalingto bind to pathways, carbohydrates, and several they can innate be a immuneviable target responses for targeted against drug pathogens. delivery Since sys‐ lectinstems. In are fact, able several to bind lectins to carbohydrates, were approved they by canFood be and a viable Drug targetAdministration for targeted for drugthat purpose. delivery systems.Information In fact, about several specific lectins carbohydrate were approved recognition by Food by andlectin Drug receptors Administration was gathered for that herein, purpose. plus Informationthe specific organs about specific where those carbohydrate lectins can recognition be found by within lectin the receptors human was body. -
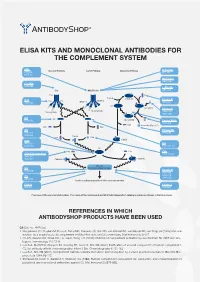
Elisa Kits and Monoclonal Antibodies for the Complement System
ELISA KITS AND MONOCLONAL ANTIBODIES FOR THE COMPLEMENT SYSTEM MBL ClassicalÊPathway LectinÊPathway AlternativeÊPathway H-Ficolin KITÊ029 RIGÊ334 HYBÊ131 M-Ficolin ABSÊ036 C1-INH HYBÊ288 L-Ficolin C1q MBL/Ficolin ABSÊ005 C1q B H2O C3Ê(H2O) FactorÊB C1s C1s C1r MASP-2 D ABSÊ002 MASP-1 HAVÊ005 C3 C3Ê(H O)ÊB 2 FactorÊD Carbohydrate Carbohydrate GAUÊ010 GAUÊ008 C2 C2 C4 C3Ê(H O)ÊBb HYBÊ050 2 Ba FactorÊBa/b HYBÊ008 C3a ProperdinÊ(FactorÊP) C2b C4a C3 Properdin HAVÊ004 HYBÊ0393 HYBÊ005 C4b2a C2a C4b C3bBb C3d HAVÊ003 C4 HYBÊ030 HYBÊ162-04 C3 C3a C3a C4b C3a/C3aÊ(desArg) HYBÊ162-02 GAUÊ013 GAUÊ017 C4b2a3b C3bBb3b C5Ê-ÊC9 C5 MembraneÊattackÊcomplex HYBÊ029 FactorÊH HYBÊ268 GAUÊ018 C9 GAUÊ020 ABSÊ004 LysisÊorÊphagocytosisÊofÊtheÊmicroorganism C5b-9 FactorÊI DIAÊ011 HYBÊ061 OverviewÊofÊtheÊcomplementÊsystem.ÊTheÊnameÊofÊtheÊcomponentÊandÊBioPortoÊDiagnostics'ÊcatalogÊnumberÊareÊshownÊinÊtheÊblueÊboxes. REFERENCES IN WHICH ANTIBODYSHOP PRODUCTS HAVE BEEN USED C2 Cat. no. HYB 050 Rooijakkers SH, Ruyken M, Roos A, Daha MR, Presanis JS, Sim RB, van Wamel WJ, van Kessel KP, van Strijp JA (2005) Immune evasion by a staphylococcal complement inhibitor that acts on C3 convertases. Nat Immunol 6:920-7. Oh KS, Kweon MH, Rhee KH, Ho Lee K, Sung HC (2003) Inhibition of complement activation by recombinant Sh-CRIT-ed1 ana- logues. Immunology 110:73-9. Laich A, Moffatt B, Wong KHN, Hickling TP, Koch C, Sim RB (2001) Purifi cation of second component of human complement, C2, by antibody affi nity chromatography. Intern J Bio-Chromatography 6:151-162. Laich A, Sim RB (2001) Complement C4bC2 complex formation: an investigation by surface plasmon resonance. Biochim Bio- phys Acta 1544:96-112. Stenbaek EI, Koch C, Barkholt V, Welinder KG (1986) Human complement component C2: production and characterization of polyclonal and monoclonal antibodies against C2. -

Table 1. Swine Proteins Identified As Differentially Expressed at 24Dpi in OURT 88/3 Infected Animals
Table 1. swine proteins identified as differentially expressed at 24dpi in OURT 88/3 infected animals. Gene name Protein ID Protein Name -Log p-value control vs A_24DPI Difference control Vs A_24DPI F8 K7GL28 Coagulation factor VIII 2.123919902 5.42533493 PPBP F1RUL6 C-X-C motif chemokine 3.219079808 4.493174871 SDPR I3LDR9 Caveolae associated protein 2 2.191007299 4.085711161 IGHG L8B0X5 IgG heavy chain 2.084611488 -4.282530149 LOC100517145 F1S3H9 Complement C3 (LOC100517145) 3.885740476 -4.364484406 GOLM1 F1S4I1 Golgi membrane protein 1 1.746130664 -4.767168681 FCN2 I3L5W3 Ficolin-2 2.937884686 -6.029483795 Table 2. swine proteins identified as differentially expressed at 7dpi in Benin ΔMGF infected animals. Gene name Protein ID Protein Name -Log p-value control vs B_7DPI Difference control Vs B_7DPI A0A075B7I5 Ig-like domain-containing protein 1.765578164 -3.480728149 ATP5A1 F1RPS8_PIG ATP synthase subunit alpha 2.270386995 3.270935059 LOC100627396 F1RX35_PIG Fibrinogen C-terminal domain-containing protein 2.211242648 3.967363358 LOC100514666;LOC102158263 F1RX36_PIG Fibrinogen alpha chain 2.337934993 3.758180618 FGB F1RX37_PIG Fibrinogen beta chain 2.411948004 4.03753376 PSMA8 F1SBA5_PIG Proteasome subunit alpha type 1.473601007 -3.815182686 ACAN F1SKR0_PIG Aggrecan core protein 1.974489764 -3.726634026 TFG F1SL01_PIG PB1 domain-containing protein 1.809215274 -3.131304741 LOC100154408 F1SSL6_PIG Proteasome subunit alpha type 1.701949053 -3.944885254 PSMA4 F2Z528_PIG Proteasome subunit alpha type 2.045768185 -4.502977371 PSMA5 F2Z5K2_PIG -

Regenerative Therapy 6 (2017) 52E64
Regenerative Therapy 6 (2017) 52e64 Contents lists available at ScienceDirect Regenerative Therapy journal homepage: http://www.elsevier.com/locate/reth Original Article Phase I clinical study of liver regenerative therapy for cirrhosis by intrahepatic arterial infusion of freshly isolated autologous adipose tissue-derived stromal/stem (regenerative) cell Yoshio Sakai a, d, Masayuki Takamura b, c, Akihiro Seki c, Hajime Sunagozaka a, c, Takeshi Terashima a, Takuya Komura c, Masatoshi Yamato a, c, Masaki Miyazawa c, Kazunori Kawaguchi a, Alessandro Nasti c, Hatsune Mochida c, Soichiro Usui b, c, * Nobuhisa Otani c, Takahiro Ochiya e, Takashi Wada d, Masao Honda a, Shuichi Kaneko a, c, a Department of Gastroenterology, Kanazawa University Hospital, Japan b Department of Cardiology, Kanazawa University Hospital, Japan c System Biology, Kanazawa University, Japan d Department of Laboratory Medicine, Kanazawa University, Japan e Division of Molecular and Cellular Medicine, National Cancer Institute, Japan article info abstract Article history: Introduction: Adipose tissue stromal cells contain a substantial number of mesenchymal stem cells. As Received 14 September 2016 such, their application to regeneration of miscellaneous impaired organs has attracted much attention. Received in revised form Methods: We designed a clinical study to investigate freshly isolated autologous adipose tissue-derived 12 November 2016 stromal/stem (regenerative) cell (ADRC) therapy for liver cirrhosis and conducted treatment in four Accepted 8 December 2016 cirrhotic patients. ADRCs were isolated from autologous subcutaneous adipose tissue obtained by the liposuction method, followed with use of the Celution system adipose tissue dissociation device. The Keywords: primary endpoint is assessment of safety one month after treatment. We also characterized the obtained Adipose tissue-derived regenerative cells Stromal cells ADRCs. -

The Role of Triggering Receptor Expressed on Myeloid Cells-2 in Inflammation
The role of triggering receptor expressed on myeloid cells-2 in inflammation Doctoral Thesis at the Medical University of Vienna for obtaining the academic degree Doctor of Philosophy (PhD) submitted by Maga. Riem Gawish Supervisor: Univ. Prof. Dr. Sylvia Knapp, PhD Laboratory of Infection Biology, Dept. of Medicine 1, Medical University Vienna Center for Molecular Medicine of the Austrian Academy of Sciences Währinger Gürtel 18-20, 1090 Vienna, Austria Vienna, 03/2015 The role of triggering receptor expressed on myeloid cells-2 in inflammation Riem Gawish Table of contents Declaration ................................................................................................................................. 5 List of Figures ............................................................................................................................ 6 List of tables ............................................................................................................................... 8 Abstract ...................................................................................................................................... 9 Kurzfassung ................................................................................................................................ 9 Publications arising from this thesis ......................................................................................... 12 Abbreviations (alphabetically ordered) .................................................................................... 13