In Lung Cancer
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Analyst Report
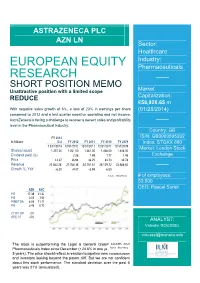
ASTRAZENECA PLC AZN LN Sector: Healthcare Industry: EUROPEAN EQUITY Pharmaceuticals RESEARCH I SHORT POSITION MEMO Market Unattractive position with a limited scope REDUCE Capitalization: €58,920.65 m With negative sales growth of 6 %, a loss of 23% in earnings per share (01/28/2014) compared to 2012 and a last quarter negative operating and net income, AstraZeneca is facing a challenge to recover a decent sales and profitability level in the Pharmaceutical industry. Country: GB FY 2013 ISIN: GB0009895292 In Millions Est FY 2012 FY 2011 FY 2010 FY 2009 Index: STOXX 600 12/31/2013 12/31/2012 12/31/2011 12/31/2010 12/31/2009 Market: London Stock Shares issued 1,257.00 1,261.00 1,361.00 1,438.00 1,448.00 Dividend yield (%) Exchange 2.26 1.99 1.77 1.48 Price 43.07 36.94 36.25 34.73 32.78 Revenue 20,462.28 21,768.38 24,151.51 25,129.22 23,588.60 oji Growth %, YoY -6.00 -9.87 -3.89 6.53 Source : Bloomberg # of employees: 53,500 CEO: Pascal Soriot AZN AVG P/E 17.38 21.32 P/S 3.05 3.56 P/EBITDA 8.04 11.11 P/B 3.45 5.72 2Y RV GR -2% OPE CF -5% ANALYST: Valentin ROUSSEL [email protected] The stock is outperforming the Legal & General Global Health and Pharmaceuticals Index since December (+24.6% in one year,Source +17.8% : Bloomberg in 5 years). The price should reflect a reaction to pipeline new introductions and investors looking beyond the patent cliff. -

Astrazeneca 17Th January 2013 Attractive Risk-Reward As New CEO Comes in Healthcare Fair Value 3440P Vs
INDEPENDENT RESEARCH AstraZeneca 17th January 2013 Attractive risk-reward as new CEO comes in Healthcare Fair Value 3440p vs. 2860p (price 3,042p) BUY vs. NEUTRAL Bloomberg AZN LN Among the big names in the pharmaceutical industry, AstraZeneca is Reuters AZN.L obviously one of the few which carries in principle one of the most 12-month High / Low (p) 3,112 / 2,591 significant upsides considering its current valuation, provided the new Market capitalisation (GBPm) 37,921 Enterprise Value (BG estimates GBPm) 39,062 CEO, Pascal Soriot, is able not only to present a comprehensive and Avg. 6m daily volume ('000 shares) 2,247 clear strategy but also to package his speech in an attractive manner Free Float 100% for the investment community to jump in as early as the beginning of 3y EPS CAGR [2012-2015] -1.2% Gearing (12/11) 8% 2013. Dividend yields (12/12e) 5.72% On 31 January 2013, Pascal Soriot is expected to present his initial YE December 12/11 12/12e 12/13e 12/14e thoughts about how to drive AstraZeneca forward and which strategy to Revenue (USDm) 33,591 28,081 27,524 27,251 EBIT(USDm) 12,795 7,921 7,818 8,334 implement. Since he took over as CEO on 1 October 2012, he has spent Basic EPS (USD) 7.33 4.81 4.58 5.04 much time meeting people within the group and also key shareholders to Core EPS (USD) 7.72 6.71 6.13 6.33 EV/Sales 1.9x 2.2x 2.2x 2.0x make the best possible assessment of the situation and to hear their EV/EBITDA 4.1x 6.0x 5.6x 5.0x expectations and hopes before presenting a roadmap. -

Astrazeneca Plc
6/15/2020 AstraZeneca - Wikipedia AstraZeneca AstraZeneca plc[3] is a British-Swedish multinational pharmaceutical and biopharmaceutical company with its global AstraZeneca plc headquarters in Cambridge, England.[4] Its R&D is concentrated in Cambridge, Gaithersburg, Maryland, and Mölndal in Sweden.[5] AstraZeneca has a portfolio of products for major disease areas including cancer, cardiovascular, gastrointestinal, infection, Type Public limited neuroscience, respiratory and inflammation.[6] company Traded as LSE: AZN (https:// The company was founded in 1999 through the merger of the www.londonstocke [7][8] Swedish Astra AB and the British Zeneca Group (itself formed xchange.com/exch by the demerger of the pharmaceutical operations of Imperial ange/searchengin Chemical Industries in 1993). Since the merger it has been among e/search.html?lang the world's largest pharmaceutical companies and has made =en&x=0&y=0&q= numerous corporate acquisitions, including Cambridge Antibody AZN) Technology (in 2006), MedImmune (in 2007), Spirogen (in 2013) NYSE: AZN (http and Definiens (by MedImmune in 2014). s://www.nyse.com/ quote/XNYS:AZN) AstraZeneca has a primary listing on the London Stock Exchange Nasdaq and is a constituent of the FTSE 100 Index. It has secondary listings Stockholm: AZN (ht on the New York Stock Exchange and the OMX exchange. tp://www.nasdaqom xnordic.com/aktier/ microsite?language Contents Id=1&Instrument=S SE3524) History FTSE 100 2000–06 Component 2007–12: The patent cliff and subsequent acquisitions ISIN GB0009895292 2013 -

How to Sustain Healthcare Transformations
Voices on transformation A marathon, not a sprint: How to sustain healthcare transformations Voices on transformation is written by experts and practitioners in McKinsey & Company’s Pharmaceuticals and Medical Products Practice. To send comments or request copies of this publication, please email us at [email protected] Editors: Gayane Gyurjyan, Ioana Parsons, Shail Thaker, Jill Willder, Carla Zwaanstra Artwork and design: Afitha de Rijk-Voeten Portrait illustrations: Allan Burch Special thanks to: Lucia Darino, Martin Dewhurst, Claudio Feser, Vincent Forlenza, Jane Griffiths, Judith Hazlewood, Nadine Mansour, Angelika Reich, Pascal Soriot, David Speiser, Kirsten Westhues, André Wyss Copyright © 2016 McKinsey & Company. All rights reserved. This publication is not intended to be used as the basis for trading in the shares of any company or for undertaking any other complex or significant financial transaction without consulting appropriate professional advisers. No part of this publication may be copied or redistributed in any form without the prior written consent of McKinsey & Company. 2 PMP Voices on transformation <Chaper title> Contents Introduction 4 Cracking the code: How successful pharma leaders 7 manage transformations A health check for pharma: Overcoming change fatigue 15 in the pharmaceutical industry Putting science at the heart of renewed purpose 26 An interview with Pascal Soriot, AstraZeneca Transforming a medical devices company into a solutions provider 34 An interview with Vincent Forlenza, Becton Dickinson Refocusing the business around patient outcomes 42 An interview with Jane Griffiths, Janssen EMEA NBS: Creating value across Novartis 48 An interview with André Wyss, Novartis Voices on digital: How pharma can win in a digital world 57 About the authors 66 3 Introduction Over the past decade, the pharmaceutical industry has been struggling to keep up with rapid and dramatic changes in the external environment. -

Annual Report 2012
Registered office and Investor relations Registrar US Depositary 2012 20-F Information Form and Report Annual AstraZeneca AstraZeneca Annual Report corporate headquarters [email protected] Equiniti Limited JPMorgan Chase & Co and Form 20-F Information 2012 AstraZeneca PLC Aspect House PO Box 64504 2 Kingdom Street UK: as above Spencer Road St Paul London W2 6BD Lancing MN 55164-0504 UK US West Sussex BN99 6DA US Tel: +44 (0)20 7604 8000 Investor Relations UK Tel: (toll free in the US) Fax: +44 (0)20 7604 8151 AstraZeneca Pharmaceuticals LP Tel: (freephone in the UK) 888 697 8018 1800 Concord Pike 0800 389 1580 Tel: (outside the US) PO Box 15437 Tel: (outside the UK) +1 (651) 453 2128 Wilmington +44 (0)121 415 7033 [email protected] DE 19850-5437 US Swedish Central Securities Tel: +1 (302) 886 3000 Depository Fax: +1 (302) 886 2972 Euroclear Sweden AB PO Box 191 SE-101 23 Stockholm Sweden Tel: +46 (0)8 402 9000 Delivering value This Annual Report is also available on through innovation our website, astrazeneca.com/ annualreport2012 Important information for readers of this Annual Report AstraZeneca Cautionary statement regarding Innovation is at the core of everything we forward-looking statements Welcome to the AstraZeneca The purpose of this Annual Report is to provide Annual Report and Form information to the members of the Company. The do at AstraZeneca – from our research 20-F Information 2012 Company and its Directors, employees, agents and (Annual Report). You will find advisers do not accept or assume responsibility to into effective new medicines to how we this Annual Report on our any other person to whom this Annual Report is website, astrazeneca.com/ shown or into whose hands it may come and any annualreport2012 such responsibility or liability is expressly disclaimed. -

What Science Can Do
What science can do April 2015 Circulating tumour DNA AstraZeneca has pioneered the use of circulating tumour DNA (ctDNA) in the diagnosis of cancer. Pieces of DNA break off from a tumour and circulate in the bloodstream where they can be analysed to give genetic information about a patient’s tumour. This allows healthcare professionals to determine the right treatment for the patient using a non-invasive blood test. At AstraZeneca, our purpose is to push the boundaries of science A message to deliver life-changing medicines. We believe the best way we can achieve this is to put science at the centre of everything we do. It from is this commitment that drives our ability to discover, develop and Pascal Soriot deliver the advancements the world needs in complex and difficult diseases like cancer, heart disease, diabetes, COPD and asthma. For us, the discovery of medicines starts with our rare combination of scientific capabilities in small molecules and biologics, immunotherapies, protein engineering technologies, oligonucleotides and devices. When combined with our strong focus on translational science and personalised healthcare, we believe that we have a unique and strong foundation for speeding novel, targeted therapies from biologic concept to care. We know that the best science doesn’t happen in isolation - it happens through collaboration. That is why we seek to work so extensively with scientific, academic and healthcare organisations around the world - from the earliest discovery work in our research labs, to our clinical trial programmes and to joint working with health care providers to achieve optimal treatment outcomes. We also take smart risks in developing and applying new technologies which help us to explore new mechanisms of action and create new types of treatments. -

Pascal Soriot Executive Director, Chief Executive Officer
Pascal Soriot Executive Director, Chief Executive Officer Pascal joined AstraZeneca as an Executive Director and Chief Executive Officer on 1 October 2012. A French national, Pascal joined AstraZeneca from Roche AG where he served as Chief Operating Officer of the company’s pharmaceuticals division since 2010. Prior to that Pascal was Chief Executive Officer of Genentech, where he was credited with leading the successful merger between the San Francisco- based biologics business and Roche. Pascal joined the pharmaceutical industry in 1986 and has worked in senior management roles in the US, Asia and Europe. Luke Miels Executive Vice President Global Portfolio & Product Strategy (GPPS) Luke started his career in 1995 with AZ in Australia where he was successively a Sales Representative and Product Manager for Plendil and Diprivan. He joined Aventis in late 2000 as Marketing and Strategic Planning Manager in Australia before being appointed Country Manager for New Zealand in 2002 and subsequently Thailand the following year. He then transferred to the USA to lead the Analytics and Commercial Effectiveness function of Aventis US. Following the Sanofi-Aventis merger he led the integration office in the US and was appointed Vice President of Sales for Metabolism at the conclusion of the merger. In 2006 he moved to Basel to join Roche as Head of Metabolism for Global Marketing. Three years later he was appointed to the role of Regional VP Asia Pacific for the Pharmaceuticals Division and joined the Leadership Team of the Pharmaceuticals Division, initially based in Shanghai and more recently in Singapore. Luke is married with three children and has an MBA from the Macquarie University, Sydney and a BSc Biology from Flinders University in Adelaide. -

Of 30 09/11/2017 About:Blank
Page 1 of 30 AstraZeneca PLC 9 November 2017 07:00 Year-To-Date and Q3 2017 Results An improved sales performance as the pipeline-driven transformation gathered pace Financial Summary YTD 2017 Q3 2017 % change % change $m $m Actual CER 1 Actual CER Total Revenue 16,688 (4) (3) 6,232 9 10 Product Sales 14,665 (9) (8) 4,882 (3) (2) Externalisation Revenue 2,023 49 50 1,350 n/m n/m Reported Operating Profit 2,991 26 16 1,149 12 9 Core Operating Profit 2 5,068 8 5 1,853 9 9 Reported Earnings Per Share (EPS) $1.34 3 (4) $0.54 (32) (33) Core EPS $2.98 (4) (7) $1.12 (15) (17) The difference in growth rates between Operating Profit and EPS included the impact of a one-off tax benefit in Q3 2016. Financial Highlights • Receding impact from losses of exclusivity: Product Sales declined by 3% (2% at CER) in the quarter • Externalisation Revenue: $2,023m, including $997m received in the quarter from the MSD 3 collaboration • Cost discipline continued: • Reported R&D costs declined by 3% (1% at CER) to $4,206m; Core R&D costs declined by 5% (2% at CER) to $3,956m • Reported SG&A costs declined by 11% (9% at CER) to $7,155m; Core SG&A costs declined by 7% (5% at CER) to $5,678m • The Company now anticipates a 2017 Core EPS performance towards the favourable end of the guidance range of a low to mid teens percentage decline Commercial Highlights The Growth Platforms grew by 3% (4% at CER) and represented 66% of Total Revenue: • Emerging Markets: 5% growth (7% at CER). -

Results Announcement: Business & Corporate
AstraZeneca PLC 24 October 2019 07:00 BST Year-to-date and Q3 2019 results Patients to benefit from further pipeline progress; sales-growth momentum driving operating leverage Review Year-to-date Product Sales growth of 13% (17% at CER1) to $17,315m included third-quarter Product Sales of $6,132m (+16%, +18% at CER). The third quarter again saw all three therapy areas and every sales region Operating & Financial Financial & Operating produce encouraging performances, including: - The continued performance of new medicines2, with sales growth in the quarter of 62% (+64% at CER) to $2,707m, including new-medicine growth in Emerging Markets of 85% (90% at CER) to $539m - Sales growth by therapy area in the quarter: Oncology +46% (+48% at CER) to $2,334m, New CVRM3 +8% (+11% at CER) to $1,113m and Respiratory +15% (+18% at CER) to $1,319m - Sales growth by region in the quarter: total Emerging Markets sales grew by 25% (29% at CER) to $2,123m, with China sales growth of 35% (40% at CER) to $1,283m, ahead of longer-term trends. US sales increased Development by 17% to $2,025m; Europe sales continued their return to growth, increasing by 1% (4% at CER) to $1,139m; Business & Corporate Japan sales increased by 31% (27% at CER) to $657m The Company today upgrades its Product Sales guidance at CER for the year. YTD 2019 Q3 2019 % change % change $m $m Actual CER Actual CER Sustainability Product Sales 17,315 13 17 6,132 16 18 Collaboration Revenue 405 3 6 274 n/m n/m Total Revenue 17,720 13 17 6,406 20 22 Reported4 Operating Profit 2,347 2 3 757 (11) (13) Core5 Operating Profit 4,891 41 42 1,880 43 41 Reported EPS6 $0.79 (11) (15) $0.23 (33) (38) Core EPS $2.61 39 38 $0.99 40 36 & Development Research Pascal Soriot, Chief Executive Officer, commenting on the results said: “With AstraZeneca growing at pace, our sales guidance has been upgraded for the second consecutive quarter. -

Acquisitions • Alliances • Financing
VOLUME 38 NUMBER 02 FEBRUARY 2020 DEAL-MAKING Acquisitions • Alliances • Financing 2020 Could Spell The End The Only Way Is Nasdaq Investors See Medtech Continuing Of Mega-Mergers, For Now To Ride Growth Wave Into 2020 PAGE LEFT BLANK INTENTIONALLY invivo.pharmaintelligence.informa.com STRATEGIC INSIGHTS FOR LIFE SCIENCES DECISION-MAKERS CONTENTS ❚ February 2020 DEAL-MAKING ACQUISITIONS ALLIANCES FINANCING 10 16 22 2020 Could Spell The End Of The Only Way Is Nasdaq Investors And Deal-Makers See Mega-Mergers, For Now MELANIE SENIOR Medtech Continuing To Ride The JOSEPH HAAS More capital, more expertise, more Growth Wave Into 2020 Industry experts expect a continued liquidity: those are the well-known ASHLEY YEO uptick in deals valued between $2bn- advantages of the US Nasdaq exchange The consensus among investors is that $10bn this year as buyers look to add over its European counterparts. Yet until medtech has been the best-performing critical mass in areas like oncology, rare recently, most European biotechs sought part of health care for the past three to disease and cell and gene therapy. a local listing before going to the US. four years, and investment levels remain Divestitures to free up capital and narrow good. Seemingly all-encompassing of late focus should continue too. has been companies’ preoccupation with digital strategies, but there is a lot more to this unique industry than just digital. 26 32 36 Biopharma M&A: Global Biopharma R&D Investing In People: Aligning Lessons From 2019, Productivity And Growth VCs And Today’s