Pascal Soriot Executive Director, Chief Executive Officer
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Medical Research Council
OFFICIAL MEDICAL RESEARCH COUNCIL 03 Should not be viewed by CEO due to Author: Sam Bartholomew Conflict of interest Should not be viewed by deputy CEO due to Conflict of interest Trade Union Side access: In part Minutes of the Council meeting held at the Chelsea Harbour Hotel on 7 March 2018 Present: Council Head Office staff Mr Donald Brydon (Chair) Ms Sam Bartholomew Sir John Savill (CEO) Professor David Lomas Professor Jonathan Bisson Dr Rob Buckle Dr John Brown Mr Hugh Dunlop Professor Doreen Cantrell Dr Declan Mulkeen Professor Chris Day Dr Tony Peatfield Professor Dame Janet Finch Mrs Helen Page Professor John Iredale Dr Rachel Quinn Mr Richard Murley Dr Frances Rawle Baroness Onora O’Neill Ms Pauline Mullin (item 9a&b only) Dr Mene Pangalos Professor Irene Tracey Guests – item 8 Dr Pauline Williams Nick Lemoine, Chair MRF Trust Angela Hind, CEO MRF Observers Ms Jenny Dibden (BEIS) Professor Fiona Watt, MRC Executive Chair Designate 1. Announcements Mr Brydon welcomed members to the last meeting of the MRC Council as currently constituted, prior to the transition to UK Research and Innovation. He introduced Professor Fiona Watt, MRC Executive Chair Designate, and Professor Jonathan Bisson who was attending his first meeting since being appointed as a Council member in the summer. He offered congratulations to those MRC-associated people whose achievements had been recognised in the New Year’s Honours: • Sir Keith Peters Sir Keith, lately Regius Professor of Physic at the University of Cambridge, was appointed Knight Grand Cross of the Order of the British Empire (GBE) for services to 1 OFFICIAL the advancement of medical science. -
Analyst Report
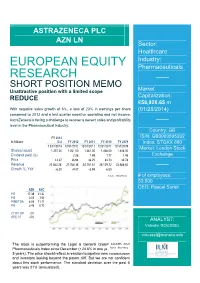
ASTRAZENECA PLC AZN LN Sector: Healthcare Industry: EUROPEAN EQUITY Pharmaceuticals RESEARCH I SHORT POSITION MEMO Market Unattractive position with a limited scope REDUCE Capitalization: €58,920.65 m With negative sales growth of 6 %, a loss of 23% in earnings per share (01/28/2014) compared to 2012 and a last quarter negative operating and net income, AstraZeneca is facing a challenge to recover a decent sales and profitability level in the Pharmaceutical industry. Country: GB FY 2013 ISIN: GB0009895292 In Millions Est FY 2012 FY 2011 FY 2010 FY 2009 Index: STOXX 600 12/31/2013 12/31/2012 12/31/2011 12/31/2010 12/31/2009 Market: London Stock Shares issued 1,257.00 1,261.00 1,361.00 1,438.00 1,448.00 Dividend yield (%) Exchange 2.26 1.99 1.77 1.48 Price 43.07 36.94 36.25 34.73 32.78 Revenue 20,462.28 21,768.38 24,151.51 25,129.22 23,588.60 oji Growth %, YoY -6.00 -9.87 -3.89 6.53 Source : Bloomberg # of employees: 53,500 CEO: Pascal Soriot AZN AVG P/E 17.38 21.32 P/S 3.05 3.56 P/EBITDA 8.04 11.11 P/B 3.45 5.72 2Y RV GR -2% OPE CF -5% ANALYST: Valentin ROUSSEL [email protected] The stock is outperforming the Legal & General Global Health and Pharmaceuticals Index since December (+24.6% in one year,Source +17.8% : Bloomberg in 5 years). The price should reflect a reaction to pipeline new introductions and investors looking beyond the patent cliff. -

Pfizer Inr 235 East 42Nd Street New York
Pfizer Inr 235 East 42nd Street New York. !W 10007-5755 March 25,2008 Steven Reynolds Director U.S. Nuclear Regulatory Commission Division of Nuclear Materials Safety 2443 Warrenville Road, STE 210 Lisle, IL 60532-4352 -Re: Parent Company Guarantee for Pharmacia Corporation (Chesterfield, MO and St. Louis, MO) Dear Mr. Reynolds: I am the Chief Executive Officer of Pfizer Inc. located at 235 East 42"d Street in New York, NY 10017. This letter is in support of this firm's use of the financial test to demonstrate financial assurance, as specified in 10 CFR Part 30. I hereby certify that Pfizer Inc. is currently a going concern, and that it possesses positive tangible net worth in the amount of $23,129,000,000. This firm is required to file a Form 10K with the U.S. Securities and Exchange Commission for the latest fiscal year. The fiscal year of this firm ends on December 3 1. I hereby certify that the content of this letter is true and correct to the best of my knowledge. panof the Board C ief Executive Officer Pfizer lnr 235 East 42nd Street New York, NY 10007-5755 March 25. 2008 Steven Reynolds Director U.S. Nuclear Regulatory Coinmission Division of Nuclear Material Safet) 2443 Warrenville Road STE 2 IO Lisle. IL 60532-4352 -Re: Financial Assurance Demonstration for Pharmacia Corporation (Chesterfield, MO and St. Louis, MO) Dear Mr. Reynolds: I am the chief financial officer of Pfizer Inc., 235 East 42'ld Street, New York. New York 10017, a corporation. This letter is in support of this firm's use of the parent company guarantee financial test to demonstrate financial assurance, as specified in IO CFR Part 30. -

Research and Development at the University of Maryland Baltimore
RESEARCH AND DEVELOPMENT AT THE UNIVERSITY OF MARYLAND BALTIMORE FISCAL YEAR 2002 ANNUAL REPORT ANNUAL REPORT TABLE OF CONTENTS Overview 1 Extramural Funding at UMB 1 Office of Research and Development Outcomes 2 Success by School 6 Areas of Strength 13 Technology Commercialization 14 New Initiatives 19 Appendixes 20 David J. Ramsay, DM, DPhil President James L. Hughes Vice President, Research & Development www.ord.umaryland.edu RESEARCH AND DEVELOPMENT AT THE UNIVERSITY OF MARYLAND BALTIMORE ANNUAL REPORT FY 02 RESEARCH AND DEVELOPMENT AT THE UNIVERSITY OF MARYLAND BALTIMORE FISCAL YEAR 2002 ANNUAL REPORT OVERVIEW The University of Maryland Baltimore reached a milestone number in extramural funding in FY 02: $305.3 million. The research conducted on campus and the hundreds of projects funded from federal, state and local governments and the private sector demonstrate UMB’s ever-increasing contribution to the life sciences and health care research fields. As an economic engine for Maryland, UMB provides access to millions of dollars of research to the business community. The commercial potential for much of this work is being actively patented and licensed. Summary of Success Principal Investigators with Awards: 617 Principal Investigators with $1 million Plus in Awards: 44 Funding Applications: 2,274 Funding Awards: 1,673 Total Dollar Amount of Extramural Funding: $305.3 Million Total Dollar Amount of Extramural Funding through ORD: $250 Million % of Total Extramural Funding: 82% Volume of Federal Awards: $142.7 Million NIH Funding Amount: $127.2 Million % of Indirect Costs: 22.8% EXTRAMURAL FUNDING AT UMB Support for extramural funding at the UMB campus has tripled in the last eight years. -

2014.10.22-110Cv00528-Pfizer-Etal
IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF DELAWARE PFIZER INC., PHARMACIA & UPJOHN ) COMPANY, PHARMACIA & UP JOHN ) COMPANY LLC, SUGEN, INC., C.P .. ) PHARMACEUTICALS INTERNATIONAL ) C.V., PFIZER PHARMACEUTICALS LLC, ) C.A. No. 10-528-GMS and PF PRISM C.V., ) ) Plaintiffs, ) ) v. ) ) MYLAN PHARMACEUTICALS INC., ) ) Defendant. ) _______________________________ ) MEMORANDUM I. INTRODUCTION In this patent infringement action, plaintiffs Pfizer Inc., Pharmacia & Upjohn Company, Pharmacia & Upjohn Company LLC, Sugen, Inc., C.P. Pharmaceuticals International C.V., Pfizer Pharmaceuticals LLC, and PF Prism C.V. (collectively, "Pfizer") allege that pharmaceutical products proposed by defendant Mylan Pharmaceuticals Inc. ("Mylan") infringe the asserted claims of the patents-in-suit. (D.I. 1.) The court held a four-day bench trial in this matter on November 26 through November 29, 2012. (D.I. 148-151.) Presently before the court are the parties' post-trial proposed findings of fact and conclusions of law concerning the validity of the patents-in-suit, specifically whether the asserted claims are invalid as obvious under 35 U.S.C. § 103. (D.I. 152, 153.) Pursuant to Federal Rule of Civil Procedure 52( a), and after having considered the entire record in this case and the applicable law, the court concludes that: (1) all asserted claims ofthe patents-in-suit are not invalid due to obviousness; and (2) Pfizer's Rule 52(c) motion is granted, and Mylan's Rule 52( c) motion is denied. These findings of fact and conclusions oflaw are set forth in further detail below. II. FINDINGS OF FACT 1 A. The Parties 1. Plaintiff Pfizer Inc. -

Optimizing Binding Kinetics in Medicinal Chemistry: Facts Or Fantasy?
Optimizing Binding Kinetics in Medicinal Chemistry: facts or fantasy? ‡ -Gon /RT kon e -G‡ /RT ‡ off ΔG on koff e -ΔGd/RT Kd e koff /kon P + L ‡ ΔG off ΔGd PL Gerhard Müller Ex-Head of Med Chem Mercachem, Nijmegen, NL Binding coordinate 11 The topic is hot, complex, and I am just an end-user & controversial “You can’t optimize koff, and you don’t need to optimize koff, you simply need to optimize Kd ! ” Head Med Chem, (top-5 Pharma), West Coast, US, Jan 2016 Optimizing Binding Kinetics in Medicinal Chemistry: facts or fantasy? ‡ -Gon /RT kon e -G‡ /RT ‡ off ΔG on koff e -ΔGd/RT Kd e koff /kon P + L ‡ ΔG off ΔGd PL Gerhard Müller Chief Scientific Officer Gotham Therapeutics, New YorkBinding coordinate 2 confidential Streetlight effect in medicinal chemistry Top-heavy distributions, rich-get-richer mechanisms 5% / 75% J. Med. Chem., 54 ,6405–6416 (2011) Vertex Pharmaceuticals, US J. Org. Chem. 73, 4443-4451 (2008) MW clogP shape clogP flatness Fsp3 flatland J. Med. Chem., 58, 2390−2405 (2015) para meta ortho J. Med. Chem., 59, 4443–4458 (2016) 3 Cheminformatics Analysis – Kinase Family Ligand and target promiscuity (ChEMBL21) Hu, Kunimoto, Bajorath Chemical Biology & Drug Design, 89, 834-845 (2017) quantitative kinome coverage 270 kinases with high-confidence data Top-10 kinases (45% of human kinome still unexplored) VEGFR2 TK 2239 erbB1 TK 1814 22.537 distinct IC50 values p38a CMGC 1509 9191 distinct scaffolds HGFR TK 1411 31.873 kinase-inhibitor combinations PI3a lipid 844 erbB-2 TK 768 GSK3b CMGC 743 SRC TK 709 Chk1 CAMK 693 -

SU14813: a Novel Multiple Receptor Tyrosine Kinase Inhibitor with Potent Antiangiogenic and Antitumor Activity
1774 SU14813: a novel multiple receptor tyrosine kinase inhibitor with potent antiangiogenic and antitumor activity Shem Patyna,1 A.Douglas Laird, 3 Dirk B.Mendel, 5 KIT, and FLT3. In cellular assays, SU14813inhibited ligand- Anne-Marie O’Farrell,2 Chris Liang,6 dependent and ligand-independent proliferation, migration, Huiping Guan,8 Tomas Vojkovsky,6 Stefan Vasile,7 and survival of endothelial cells and/or tumor cells express- B Xueyan Wang,9 Jeffrey Chen,1 Maren Grazzini,1 ing these targets. SU14813inhibited VEGFR-2, PDGFR- , Cheng Y.Yang, 10 Joshua O˝.Haznedar, 5 and FLT3phosphorylation in xenograft tumors in a dose- 4 1 and time-dependent fashion. The plasma concentration Juthamas Sukbuntherng, Wei-Zhu Zhong, in vivo 2 1 required for target inhibition was estimated to be Julie M.Cherrington, and Dana Hu-Lowe 100 to 200 ng/mL. Used as monotherapy, SU14813 1Pfizer Global Research and Development; 2Phenomix Corp., exhibited broad and potent antitumor activity resulting in San Diego, California; 3Exelixis, Inc.; 4Celera Genomics, Inc., regression, growth arrest, or substantially reduced South San Francisco, California; 5Chiron Corp., Emeryville, growth of various established xenografts derived from 6 7 California; The Scripps Research Institute; The Burnham human or rat tumor cell lines. Treatment in combination Institute, La Jolla, California; 8AstraZeneca PLC, Waltham, Massachusetts; 9Metabolex, Inc., Hayward, California; and with docetaxel significantly enhanced both the inhibition 10Gilead Sciences, Inc., Foster City, California of primary tumor growth and the survival of the tumor- bearing mice compared with administration of either agent alone. In summary, SU14813inhibited target RTK Abstract activity in vivo in association with reduction in angio- Receptor tyrosine kinases (RTK), such as vascular endo- genesis, target RTK-mediated proliferation, and survival thelial growth factor receptor (VEGFR), platelet-derived of tumor cells, leading to broad and potent antitumor growth factor receptor (PDGFR), stem cell factor receptor efficacy. -

Documenting the Biotechnology Industry in the San Francisco Bay Area
Documenting the Biotechnology Industry In the San Francisco Bay Area Robin L. Chandler Head, Archives and Special Collections UCSF Library and Center for Knowledge Management 1997 1 Table of Contents Project Goals……………………………………………………………………….p. 3 Participants Interviewed………………………………………………………….p. 4 I. Documenting Biotechnology in the San Francisco Bay Area……………..p. 5 The Emergence of An Industry Developments at the University of California since the mid-1970s Developments in Biotech Companies since mid-1970s Collaborations between Universities and Biotech Companies University Training Programs Preparing Students for Careers in the Biotechnology Industry II. Appraisal Guidelines for Records Generated by Scientists in the University and the Biotechnology Industry………………………. p. 33 Why Preserve the Records of Biotechnology? Research Records to Preserve Records Management at the University of California Records Keeping at Biotech Companies III. Collecting and Preserving Records in Biotechnology…………………….p. 48 Potential Users of Biotechnology Archives Approaches to Documenting the Field of Biotechnology Project Recommendations 2 Project Goals The University of California, San Francisco (UCSF) Library & Center for Knowledge Management and the Bancroft Library at the University of California, Berkeley (UCB) are collaborating in a year-long project beginning in December 1996 to document the impact of biotechnology in the Bay Area. The collaborative effort is focused upon the development of an archival collecting model for the field of biotechnology to acquire original papers, manuscripts and records from selected individuals, organizations and corporations as well as coordinating with the effort to capture oral history interviews with many biotechnology pioneers. This project combines the strengths of the existing UCSF Biotechnology Archives and the UCB Program in the History of the Biological Sciences and Biotechnology and will contribute to an overall picture of the growth and impact of biotechnology in the Bay Area. -

Scientific Insights in a Pandemic COVID-19 National Core Studies Symposium 24 June 2021
Scientific Insights in a Pandemic COVID-19 National Core Studies Symposium 24 June 2021 Agenda Session title and speaker 09:00 Opening keynote: A year of accelerated science and impacts Jeremy Farrar, Wellcome Trust 09.10 UK COVID Science, the public and the media • Fiona Fox, Science Media Centre • Chris Monk, public representative 09.20 Scientific impact on the NHS and patients Mark Toshner, Cambridge University Hospitals NHS Foundation Trust 09.30 Plenary keynote: What we have learned from COVID? Patrick Vallance, Chief Scientific Advisor UK Government 09.50 National Core Studies – impact and future potential • Transmission and environment – Andrew Curran • Longitudinal health and wellbeing – Nish Chaturvedi and Jonathan Sterne • Immunity – Paul Moss • Epidemiology and Surveillance – Ian Diamond • Vaccines Trials Infrastructure – Divya Chadha Manek • Therapeutics Trials Infrastructure – Patrick Chinnery • Data and Connectivity – Andrew Morris Chaired by Anne Johnson Q&A 11.10 Break 11.20 Lightning talks - Scientific Impact of UK COVID science 11.20 Group 1 Group 2 • Anne-Marie Docherty, University of • Dinesh Aggarwal, University of Edinburgh Cambridge • Marion Mafham, Nuffield Department of • Helen Parry, NIHR Population Health, University of Oxford • Joshua Elliott, Imperial College London • Muzz Hanifa, Newcastle University • William Hulme, Oxford DataLab • Cameron Razieh, University of Leicester Q&A Q&A 12.10 Preparing for the next pandemic • Mene Pangalos, AstraZeneca • Gabriel Leung, University of Hong Kong • Lynda Stuart, Bill & Melinda Gates Foundation • Further speakers TBC 12:35 International Guest lecture - Opportunities for global collaborative open science Keynote speaker TBC Fireside chat with Ottoline Leyser, Chief Executive, UK Research and Innovation 12:55 Closing remarks Patrick Vallance, Government Chief Scientific Adviser 13:00 End . -

Pfizer, Inc., Letter of Support for Parent
Ptizer Inc 235 East 42nd Street New York, NY 1OOl7-5755 March 25,2008 Steven Reynolds Director U.S.Nuclear Regulatory Commission Division of Nuclear Material Safety 2443 Warrenville Road, STE 2 10 Lisle, IL 60532-4352 -Re: Parent Company Guarantee for Pharmacia & Upjohn Co. (Kalamazoo, MI) Dear Mr. Reynolds: I am the Chief Executive Officer of Pfizer Inc. located at 235 East 42ndStreet in New York, NY 10017. This letter is in support of this firm's use of the financial test to demonstrate financial assurance, as specified in 10 CFR Part 30. I hereby certify that Pfizer Inc. is currently a going concern, and that it possesses positive tangible net worth in the amount of $23,129,000,000. This firm is required to file a Form 10K with the U.S. Securities and Exchange Commission for the latest fiscal year. The fiscal year of this firm ends on December 3 1. I hereby certify that the content of this letter is true and correct to the best of my knowledge. ofthe ~oard Chief Executive Officer RECEIVED MAR 2 8 2008 .' . Pfizer Inr 235 East 42nd Street New lork. NY 10007-5755 March 25, 2008 Steven Reynolds Director U .S . N uc I ear Reg u la t ory C om m i ss i on Division of Nuclear Material Safet) 2443 Warrenville Road, STE 2 IO Lisle, IL 60532-4352 -Re: Financial Assurance for Pharmacia & Upjohn Company LLC (Kalainazoo, MI) Dear Mr. Reynolds: 1 am the chief financial officer of Pfizer Inc.. 235 East 42'IdStreet, New York. -

Astrazeneca 17Th January 2013 Attractive Risk-Reward As New CEO Comes in Healthcare Fair Value 3440P Vs
INDEPENDENT RESEARCH AstraZeneca 17th January 2013 Attractive risk-reward as new CEO comes in Healthcare Fair Value 3440p vs. 2860p (price 3,042p) BUY vs. NEUTRAL Bloomberg AZN LN Among the big names in the pharmaceutical industry, AstraZeneca is Reuters AZN.L obviously one of the few which carries in principle one of the most 12-month High / Low (p) 3,112 / 2,591 significant upsides considering its current valuation, provided the new Market capitalisation (GBPm) 37,921 Enterprise Value (BG estimates GBPm) 39,062 CEO, Pascal Soriot, is able not only to present a comprehensive and Avg. 6m daily volume ('000 shares) 2,247 clear strategy but also to package his speech in an attractive manner Free Float 100% for the investment community to jump in as early as the beginning of 3y EPS CAGR [2012-2015] -1.2% Gearing (12/11) 8% 2013. Dividend yields (12/12e) 5.72% On 31 January 2013, Pascal Soriot is expected to present his initial YE December 12/11 12/12e 12/13e 12/14e thoughts about how to drive AstraZeneca forward and which strategy to Revenue (USDm) 33,591 28,081 27,524 27,251 EBIT(USDm) 12,795 7,921 7,818 8,334 implement. Since he took over as CEO on 1 October 2012, he has spent Basic EPS (USD) 7.33 4.81 4.58 5.04 much time meeting people within the group and also key shareholders to Core EPS (USD) 7.72 6.71 6.13 6.33 EV/Sales 1.9x 2.2x 2.2x 2.0x make the best possible assessment of the situation and to hear their EV/EBITDA 4.1x 6.0x 5.6x 5.0x expectations and hopes before presenting a roadmap. -

Astrazeneca Plc
6/15/2020 AstraZeneca - Wikipedia AstraZeneca AstraZeneca plc[3] is a British-Swedish multinational pharmaceutical and biopharmaceutical company with its global AstraZeneca plc headquarters in Cambridge, England.[4] Its R&D is concentrated in Cambridge, Gaithersburg, Maryland, and Mölndal in Sweden.[5] AstraZeneca has a portfolio of products for major disease areas including cancer, cardiovascular, gastrointestinal, infection, Type Public limited neuroscience, respiratory and inflammation.[6] company Traded as LSE: AZN (https:// The company was founded in 1999 through the merger of the www.londonstocke [7][8] Swedish Astra AB and the British Zeneca Group (itself formed xchange.com/exch by the demerger of the pharmaceutical operations of Imperial ange/searchengin Chemical Industries in 1993). Since the merger it has been among e/search.html?lang the world's largest pharmaceutical companies and has made =en&x=0&y=0&q= numerous corporate acquisitions, including Cambridge Antibody AZN) Technology (in 2006), MedImmune (in 2007), Spirogen (in 2013) NYSE: AZN (http and Definiens (by MedImmune in 2014). s://www.nyse.com/ quote/XNYS:AZN) AstraZeneca has a primary listing on the London Stock Exchange Nasdaq and is a constituent of the FTSE 100 Index. It has secondary listings Stockholm: AZN (ht on the New York Stock Exchange and the OMX exchange. tp://www.nasdaqom xnordic.com/aktier/ microsite?language Contents Id=1&Instrument=S SE3524) History FTSE 100 2000–06 Component 2007–12: The patent cliff and subsequent acquisitions ISIN GB0009895292 2013