Root Nodule Structure in Chamaecytisus Podolicus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fruits and Seeds of Genera in the Subfamily Faboideae (Fabaceae)
Fruits and Seeds of United States Department of Genera in the Subfamily Agriculture Agricultural Faboideae (Fabaceae) Research Service Technical Bulletin Number 1890 Volume I December 2003 United States Department of Agriculture Fruits and Seeds of Agricultural Research Genera in the Subfamily Service Technical Bulletin Faboideae (Fabaceae) Number 1890 Volume I Joseph H. Kirkbride, Jr., Charles R. Gunn, and Anna L. Weitzman Fruits of A, Centrolobium paraense E.L.R. Tulasne. B, Laburnum anagyroides F.K. Medikus. C, Adesmia boronoides J.D. Hooker. D, Hippocrepis comosa, C. Linnaeus. E, Campylotropis macrocarpa (A.A. von Bunge) A. Rehder. F, Mucuna urens (C. Linnaeus) F.K. Medikus. G, Phaseolus polystachios (C. Linnaeus) N.L. Britton, E.E. Stern, & F. Poggenburg. H, Medicago orbicularis (C. Linnaeus) B. Bartalini. I, Riedeliella graciliflora H.A.T. Harms. J, Medicago arabica (C. Linnaeus) W. Hudson. Kirkbride is a research botanist, U.S. Department of Agriculture, Agricultural Research Service, Systematic Botany and Mycology Laboratory, BARC West Room 304, Building 011A, Beltsville, MD, 20705-2350 (email = [email protected]). Gunn is a botanist (retired) from Brevard, NC (email = [email protected]). Weitzman is a botanist with the Smithsonian Institution, Department of Botany, Washington, DC. Abstract Kirkbride, Joseph H., Jr., Charles R. Gunn, and Anna L radicle junction, Crotalarieae, cuticle, Cytiseae, Weitzman. 2003. Fruits and seeds of genera in the subfamily Dalbergieae, Daleeae, dehiscence, DELTA, Desmodieae, Faboideae (Fabaceae). U. S. Department of Agriculture, Dipteryxeae, distribution, embryo, embryonic axis, en- Technical Bulletin No. 1890, 1,212 pp. docarp, endosperm, epicarp, epicotyl, Euchresteae, Fabeae, fracture line, follicle, funiculus, Galegeae, Genisteae, Technical identification of fruits and seeds of the economi- gynophore, halo, Hedysareae, hilar groove, hilar groove cally important legume plant family (Fabaceae or lips, hilum, Hypocalypteae, hypocotyl, indehiscent, Leguminosae) is often required of U.S. -

Patterns of Flammability Across the Vascular Plant Phylogeny, with Special Emphasis on the Genus Dracophyllum
Lincoln University Digital Thesis Copyright Statement The digital copy of this thesis is protected by the Copyright Act 1994 (New Zealand). This thesis may be consulted by you, provided you comply with the provisions of the Act and the following conditions of use: you will use the copy only for the purposes of research or private study you will recognise the author's right to be identified as the author of the thesis and due acknowledgement will be made to the author where appropriate you will obtain the author's permission before publishing any material from the thesis. Patterns of flammability across the vascular plant phylogeny, with special emphasis on the genus Dracophyllum A thesis submitted in partial fulfilment of the requirements for the Degree of Doctor of philosophy at Lincoln University by Xinglei Cui Lincoln University 2020 Abstract of a thesis submitted in partial fulfilment of the requirements for the Degree of Doctor of philosophy. Abstract Patterns of flammability across the vascular plant phylogeny, with special emphasis on the genus Dracophyllum by Xinglei Cui Fire has been part of the environment for the entire history of terrestrial plants and is a common disturbance agent in many ecosystems across the world. Fire has a significant role in influencing the structure, pattern and function of many ecosystems. Plant flammability, which is the ability of a plant to burn and sustain a flame, is an important driver of fire in terrestrial ecosystems and thus has a fundamental role in ecosystem dynamics and species evolution. However, the factors that have influenced the evolution of flammability remain unclear. -

Chamaecytisus Proliferus (Cytisus Palmensis, Cytisus Proliferus, Chamaecytisus Palmensis) Fabaceae
Chamaecytisus proliferus (Cytisus palmensis, Cytisus proliferus, Chamaecytisus palmensis) Fabaceae Canary Islands Am: Tree lucern Eng: Tagasaste, Tree lucerne Propagation Ecology Seedlings. Widely planted in the Mediterranean area and Australia as a browse and forage Seed shrub. Also grown in USA, Latin America, About 45,000 seed per kg. India and some other African countries. Treatment: Inoculate the seed with cow‑ Recorded as invasive in Australia. pea inoculums. Immerse the seed in hot Introduced to Ethiopia in the 1980s. It is water for a minute. growing well in moist and dry highlands and could be successful in Moist and Wet Storage: Seeds can be stored for 4–5 Weyna Dega and Dega agroclimatic zones, years. 1,700–3,300 m. It grows best in high‑ Management rainfall cool highlands. Plant seedlings when two months old. Uses Harvest fodder frequently by pruning to Firewood, fodder (leaves, pods), bee forage, encourage low, bushy and readily accessible mulch, nitrogen fixation, soil conservation, re‑growth and to reduce the amount of soil improvement, windbreak, live fence. woody stems. Description Remarks An evergreen shrub or small tree to about Tolerates drought, repeated browsing or 6 m. LEAVES: Compound with 3 stalked harvesting once established. The leaves are leaflets, the central largest to 7 cm, narrow excellent fodder with a high food value. and oblong to a pointed tip, narrowed to Small birds are fond of the seeds. Relatively the base, stalk to 2 cm long. FLOWERS: free of pests and diseases, this species needs White. FRUIT: Hairy pods to 5 cm long to be protected from grazers at first. -

Species of Septoria on the Fabaceae, Subfamily Faboideae, Tribe Genisteae
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Sydowia Jahr/Year: 1992 Band/Volume: 44 Autor(en)/Author(s): Farr David F. Artikel/Article: Species of Septoria on the Fabaceae, subfamily Faboideae, tribe Genisteae. 13-31 ©Verlag Ferdinand Berger & Söhne Ges.m.b.H., Horn, Austria, download unter www.biologiezentrum.at Species of Septoria on the Fabaceae, subfamily Faboideae, tribe Genisteae D. F. Farr Systematic Botany and Mycology Laboratory Agriculture Research Service, U.S.D.A. Beltsville, Maryland Farr, D. F. (1992). Species of Septoria on the Fabaceae, subfamily Faboideae, tribe Genisteae. - Sydowia 44: 13-31. Fourteen taxa of Septoria that have been described on members of the Fabaceae, subfamily Faboideae, tribe Genisteae were studied. Seven taxa are accepted including the new taxa Septoria sulphurei, S. cytisi var. alpini, and S. cytisi var. petteriae. Conidial morphology, hosts and lesion morphology were found to be valuable in separating taxa. Illustrations and a key to the accepted taxa are included. Keywords: taxonomy, coelomycetes, pycnidial fungi, Deuteromycotina. The Fabaceae contains many economically important taxa, sev- eral of which have been reported to be infected with species of Septoria, including S. astragali Roberge in Desmaz. on Astragalus, S. glycines Hemi on Glycine, S. fulvescens Sacc. on Lathyrus, and S. pisi Westend, on Pisum. This paper covers the taxonomy of the Septoria species that have been reported on members of the Fabaceae, subfamily Faboideae, tribe Genisteae, as part of a larger study of the approximately 160 species of Septoria reported on the Fabaceae. The tribe Genisteae was chosen primarily because the type of the genus, Septoria cytisi Desmaz., occurs on Cytisus, one of the genera in this tribe. -

Two New Chamaecytisus Species (Leguminosae-Papilionoideae) from Albania, with an Overview on the Ch. Ratisbonensis and Ch. Eriocarpus Species Groups
DOI: 10.17110/StudBot.2016.47.1.163 Studia bot. hung. 47(1), pp. 163–178, 2016 TWO NEW CHAMAECYTISUS SPECIES (LEGUMINOSAE-PAPILIONOIDEAE) FROM ALBANIA, WITH AN OVERVIEW ON THE CH. RATISBONENSIS AND CH. ERIOCARPUS SPECIES GROUPS Dániel Pifkó* and Zoltán Barina Department of Botany, Hungarian Natural History Museum H–1431 Budapest, Pf. 137, Hungary; *pifk [email protected] Pifk ó, D. & Barina, Z. (2016): Two new Chamaecytisus species (Leguminosae-Papilionoideae) from Albania, with an overview on the Ch. ratisbonensis and Ch. eriocarpus species groups. – Stu- dia bot. hung. 47(1): 163–178. Abstract: Two new species, Chamaecytisus korabensis and Ch. pseudojankae, both from Albania, are described and illustrated here. Th e relationship of the new taxa to Ch. ratisbonensis and Ch. eriocarpus agg. is discussed. Ch. korabensis is an endemic species of eastern Albania and related to the Eastern European Ch. ratisbonensis agg. Ch. pseudojankae is an endemic species of the Th atë and Galičica Mountain ranges, and morphologically it is between Ch. austriacus and Ch. eriocarpus agg. Additionally, the names Cytisus absinthioides and C. pygmaeus are lectotypifi ed. Key words: Fabaceae, endemic species, lectotype, taxonomy INTRODUCTION Th e genus Chamaecytisus is distributed from the Canary Islands to Anatolia, occurring throughout the entire Mediterranean region and Europe except for the western and northern parts. Up to now, approximately 350 taxa have been de- scribed in the genus Chamaecytisus Link (incl. ca 120 species). Due to taxonomic and nomenclatural reasons more than 750 related names are present in the litera- ture (Pifkó 2015). Th e treatment of Chamaecytisus taxa is highly variable in monographs, thus the clear elucidation of the known taxa, based on type material, is necessary when describing new species. -

Historical Aspects of the Origin and Distribution of Tagasaste (Chamaecytisus Proliferus (L
J. Adelaide Bot.Gard. 14(1): 67-76 (1991) HISTORICAL ASPECTS OF THE ORIGIN AND DISTRIBUTION OF TAGASASTE (CHAMAECYTISUS PROLIFERUS (L. FIL.)LINK SSP. PALMENSIS (CHRIST)KUNKEL), A FODDER TREE FROM THE CANARY ISLANDS J. Francisco-Ortega & M.T. Jackson Plant Genetics Group, School of Biological Sciences, University of Birmingham, Edgbaston, Birmingham,B15 2TT,United Kingdom and A. Santos-Guerra & M. Fernández-Galván Jardín de Aclimatación de La Orotava, Puerto de La Cruz, Tenerife, Canary Islands, Spain Abstract Chamaecytisus proliferus (L. fil.)Link (Fabaceae: Genisteae) forms a taxonomic complex which is endemic to El Hierro, La Palma, La Gomera, Tenerife and Gran Canaria in the Canary Island archipelago. Forms from La Palma are popularly known as "tagasaste" whereas those from the rest of the archipelago are commonly called "escobón". Tagasaste is the only form which is broadly cultivated in the Canary Islands, and since the late 19th century in New Zealand and Australia. It has also become naturalized in Australia (South Australia, New South Wales, Victoria and Tasmania), Java, the Hawaiian Islands, California, Portugal, North Africa, Kenya, Tanzania and South Africa. Dr Victor Pérez, a medical practitioner from La Palma, introduced tagasaste as a fodder tree from La Palma to Tenerife by the middle 19th century. Early introductions of tagasaste from the Canary Islands in the Pacific region confirm the importance of the Royal Botanic Gardens, Kew and the Botanic Gardens of Adelaide in the distribution of this exotic species in this region during the last c,entury. Introduction The endemic Chamaecytisus from the Canary Islands comprises a taxonomic complex found in the islands of El Hierro, La Palma, La Gomera, Tenerife and Gran Canaria. -

Broom Management
Reprinted fromPlant Plant Protection Protection Quarterly Quarterly Vol.15(4) Vol.15(4) 2000 2000 131 Broom management Proceedings of a workshop held at Ellerston and Moonan on 16–17 November 1998 This is part of a series of workshops sponsored by the Co-operative Research Centre for Weed Management Systems Editors: A.W. Sheppard and J.R. Hosking Broom management CRC eed management systems 132 Plant Protection Quarterly Vol.15(4) 2000 CRC Co-operative Research Centre for eed Weed Management Systems management Established and supported under the Australian Government’s systems Co-operative Research Centres Program MISSION STATEMENT The CRC is committed to increasing the sustainability of agriculture and protecting the natural environment by developing ecologically sound, cost effective weed manage- ment systems. OBJECTIVES • To reduce the impact of weeds on farm productivity and profitability by developing sustainable management programs that optimize the integration of chemical, bio- logical and ecological approaches for annual crop and pasture systems in the crop- ping zone of southern Australia. • To develop practical integrated weed management systems that reduce weed infes- tation, protect the environment and enhance sustainability and productivity of Aus- tralian temperate perennial pasture ecosystems. • To develop integrated strategies for the sustainable management of weeds invading natural ecosystems in temperate Australia, in order to maintain biological diversity of native flora and fauna and to prevent further degradation of natural habitats. • To implement a suite of weed science and weed management education programs which, for the first time in Australia, offers a coordinated approach to educating undergraduates, postgraduates, professional land and natural resource managers, and the community. -
Diversity, Rarity and the Evolution and Conservation of the Canary Islands Endemic Flora
Anales del Jardín Botánico de Madrid Vol. 65(1): 25-45 enero-junio 2008 ISSN: 0211-1322 Diversity, rarity and the evolution and conservation of the Canary Islands endemic flora by J. Alfredo Reyes-Betancort1, Arnoldo Santos Guerra1, I. Rosana Guma1, Christopher J. Humphries2 & Mark A. Carine2,3 1 Unidad de Botánica Aplicada, Instituto Canario de Investigaciones Agrarias, Jardín de Aclimatación de La Orotava, c/ Retama n.º 2, 38400 Puerto de La Cruz, Santa Cruz de Tenerife, Spain 2 Department of Botany, The Natural History Museum, Cromwell Road, London SW7 5BD, United Kingdom 3Author for correspondence: [email protected] Abstract Resumen Reyes-Betancort, J.A., Santos Guerra, A., Guma, I.R., Humphries, Reyes-Betancort, J.A., Santos Guerra, A., Guma, R., Humphries, C.J. & Carine, M.A. 2008. Diversity, rarity and the evolution and C.J. & Carine, M.A. 2008. Diversidad, rareza, evolución y con- conservation of the Canary Islands endemic flora. Anales Jard. servación de la flora endémica de las Islas Canarias. Anales Jard. Bot. Madrid 65(1): 25-45. Bot. Madrid 65(1): 25-45 (en inglés). The endemic vascular flora of the Canary Islands comprises over La flora vascular endémica de las Islas Canarias comprende unos 680, taxa collectively accounting for more than 50% of the total 680 táxones, lo que viene a representar más del 50% de la flora na- native flora. To investigate geographical patterns of diversity tiva. Con objeto de investigar patrones geográficos de diversidad within the endemic flora, distribution data from published en la flora endémica, se recopilaron los datos publicados que, jun- sources together with other field observation and herbarium to con otras observaciones de campo y datos de herbario, sirvieron data were used to compile a data matrix comprising the distrib- para completar una matriz de datos que abarca la distribución de utions of ca. -

Genista Monspessulana (L.) L
Selection and Testing of Biological Control Agents for Control of French Broom Genista monspessulana (L.) L. Johnson Oregon Department of Agriculture ODA project 801 GR Final Report Andy Sheppard Project Leader CRC Weed Management Systems/CSIRO Entomology CSIRO European Laboratory-based project Campus International de Baillarguet 34980 Montferrier-sur-Lez, France Acknowledgements Australian Cooperative Research Centre for Weed Management Systems who jointly funding this work. Janine Lloyd (CRC) for use of her data from Australia and Europe and data jointly collected in Europe, Thierry Thomann and Sylvie Agret (CSIRO) for assistance with data collection and processing and Janine Vitou (CSIRO) for assistance with mounting specimens and arranging to have them identified. Biological control of French broom, Genista monspessulana ODA/CDF Final Report 2000 Table of Contents Page Executive summary 3 Task 1: Literature Review 5 Task 2: Selection and collection of test plant materials 12 Task 3: Testing protocols 18 Task 4: Research planning 21 Task 5: Identification of potential biological control agents 24 Task 6: Laboratory and field-testing 30 Task 7: Literature and reports 33 References 33 Appendix 1: IBI proposal 38 2 Biological control of French broom, Genista monspessulana ODA/CDF Final Report 2000 Executive summary This project final report addresses tasks as outlined in the Task List included in ODA 801 GR grant agreement between Oregon Department of Agriculture (ODA) and CSIRO Entomology signed on the 8th September 2000 with completion on or before 31st December 2000 on the selection and testing of biological control agents for control of French broom Genista monspessulana (L.) L. Johnson. -
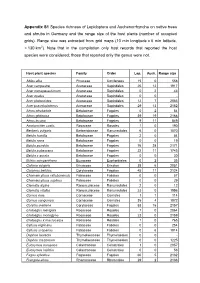
Appendix S1 Species Richness of Lepidoptera and Auchenorrhyncha on Native Trees and Shrubs in Germany and the Range Size of the Host Plants (Number of Occupied Grids)
Appendix S1 Species richness of Lepidoptera and Auchenorrhyncha on native trees and shrubs in Germany and the range size of the host plants (number of occupied grids). Range size was extracted from grid maps (10 min longitude x 6 min latitude, ≈ 130 km 2). Note that in the compilation only host records that reported the host species were considered; those that reported only the genus were not. Host plant species Family Order Lep. Auch. Range size Abies alba Pinaceae Coniferales 15 0 558 Acer campestre Aceraceae Sapindales 25 12 1917 Acer monspessulanum Aceraceae Sapindales 0 2 43 Acer opalus Aceraceae Sapindales 0 0 - Acer platanoides Aceraceae Sapindales 12 7 2083 Acer pseudoplatanus Aceraceae Sapindales 29 13 2152 Alnus alnobetula Betulaceae Fagales 0 2 54 Alnus glutinosa Betulaceae Fagales 39 19 2168 Alnus incana Betulaceae Fagales 9 11 849 Amelanchier ovalis Rosaceae Rosales 1 0 190 Berberis vulgaris Berberidaceae Ranunculales 6 0 1070 Betula humilis Betulaceae Fagales 2 0 54 Betula nana Betulaceae Fagales 0 0 19 Betula pendula Betulaceae Fagales 76 28 2171 Betula pubescens Betulaceae Fagales 22 11 1745 Betula x aurata Betulaceae Fagales 0 0 30 Buxus sempervirens Buxaceae Euphorbiales 0 2 35 Calluna vulgaris Ericaceae Ericales 38 6 2051 Carpinus betulus Corylaceae Fagales 45 11 2124 Chamaecytisus ratisbonensis Fabaceae Fabales 0 0 57 Chamaecytisus supinus Fabaceae Fabales 0 0 29 Clematis alpina Ranunculaceae Ranunculales 2 0 12 Clematis vitalba Ranunculaceae Ranunculales 23 0 1556 Cornus mas Cornaceae Cornales 1 1 114 Cornus sanguinea -

Microsoft Word
Subfamily Faboideae Scientific Classification Kingdom: Plantae Subkingdom: Tracheobionta (Vascular plants/Piante vascolari) Superdivision: Spermatophyta (Seed plants/Piante con semi) Division: Magnoliophyta (Flowering plants/Piante con fiori) Class: Rosopsida Batsch, 1788 Subclass: Rosidae Takht., 1967 SuperOrder: Fabanae R. Dahlgren ex Reveal, 1993 Order: Fabales Family: Fabaceae o Papilionacee Subfamily: Faboideae o Papilionoideae Faboideae is a subfamily of the flowering plant family Fabaceae . An acceptable alternative name for the subfamily is Papilionoideae . This subfamily is widely distributed and members are adapted to a wide variety of environments. Faboideae may be trees, shrubs or herbs. The flowers are classically pea shaped and root nodulation is very common. Flowers: Zygomorphic, papilionaceous; hypan-thium present; petals 5 [1 banner or standard petal outermost, 2 free lateral wing petals, and 2 petals fused to form the keel]; stamens 10, usually diadelphous (9 connate, 1 free), sometimes monadelphous or all free Inflorescences: Racemes, spikes, or heads Fruits: Diverse legumes Seeds: Without endosperm; lacking pleurogram Habit: Mostly herbs, some trees and shrubs; temperate, subtropical, and tropical Leaves: Usually pinnately compound, sometimes palmately compound, rarely simple, alternate, with stipules The belonging genera to the Faboideae family are: • Abrus • Craspedolobium • Kummerowia • Podalyria • Acosmium • Cratylia • Lablab • Podocytisus • Adenocarpus • Crotalaria • Laburnum • Poecilanthe • Adenodolichos • Cruddasia -
Chamaecytisus Palmensis Star Performer Status Chamaecytisus Palmensis, Called Tagasaste Or Tree Lucerne, Is in the Legume Family (Fabaceae)
Chamaecytisus palmensis Star Performer Status Chamaecytisus palmensis, called tagasaste or tree lucerne, is in the legume family (Fabaceae). Tagasaste is a significant star performer in New Zealand because it flowers from late winter through early spring when little else is flowering for bees. Tagasaste starts flowering as early as June, peaking from August to September, when bees are emerging from their winter rest to build up colonies. Each shrub/tree has prolific floral displays. Flowering can continue for up to four months. Cultivated and naturalised tagasaste are important multi-function plants much used by farmers and other landowners, so it is abundant and Figure 1. The floral display of tagasaste branches are composed common everywhere, making it a plentiful and available forage plant for honey of densely packed flowers in various stages of opening. bees. Introduction One of the puzzling features of tagasaste is that we often find few honey bees foraging at one time on a tree. In other words, it is not what we call a ‘buzz plant’ with a frenzy of numerous honey bees buzzing loudly and covering the whole tree. This is because the flowers are in several different stages of opening along the branch and the flowers have a complex structure that challenge honey bees to gain access to pollen and nectar in the young flowers. The flowers of tagasaste are ‘closed-access’ flowers in the shape of the familiar Figure 3. This honey bee may be collecting nectar at the back of ‘flag blossom’ type also found in other members of the legume family such as the flower through a hole made by bumble bees.