Tao Masters: Tradition, Experience and Ethnography*
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Unveil the Mystery of Medical Chi Gong by Dr. Kevin Chen
SPORTS / RECREATION / GENERAL RECREATION Unveil the mystery of Medical Chi Gong by Dr. Kevin Chen January 27, 2010 9:18 PM MST The ancient healing art Chi Gong (Qigong) was practiced by many cultures, i.e. Indian Prana, Japaness Ki, Germany OD, and Hawaiian Mana. In China, the written evidence of Chi Gong practice was dated back to 600 BCE on a jade pendant. To the majority of the world’s population, Chi Gong remains a mystery, even in the eyes of many Chinese. The medical Chi Gong is even more difficult to comprehend. Dr. Kevin Chen on Chi Gong (copied from the speech video) Professor Kevin Chen of University of Maryland School of Medicine, a veteran Chi Gong practitioner and science researcher, was invited to deliver an overview on the Medical Chi Gong at the National Institutes of Health (NIH) in Oct 2009. With his profound knowledge on Chi Gong, the Traditional Chinese Medicine (TCM), and modern scientific Chi Gong research, the speech was fascinating. With the long history of Chi Gong practices, Chinese have developed thousands of Chi Gong styles and forms. The other names for Chi Gong are Tu-na, Dao-yin, An-qiao, Xiu-lian, Jing-zuo, Yang-sheng, Cun-si, Guan-xiang, and Xing-qi. Dr. Chen classified all Chinese Chi Gong practices into 5 different categories: Confucian, Buddhist, Daoism (Taoism), Medical, and Martial-arts (e.g. Taichi). Citing from a Chinese university textbook Chinese Medical Qigong, Dr. Chen defines Chi Gong as “ Body-Mind exercise or techniques that integrated body, breathe and mind adjustment into Oneness “. -
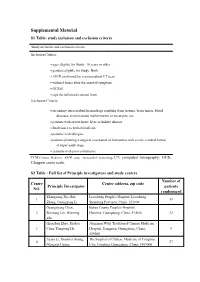
Supplemental Material S1 Table: Study Inclusion and Exclusion Criteria
Supplemental Material S1 Table: study inclusion and exclusion criteria Study inclusion and exclusion criteria Inclusion Criteria: ages eligible for Study: 18 years or older genders eligible for Study: Both AICH confirmed by craniocerebral CT scan within 6 hours after the onset of symptom GCS≥6 sign the informed consent form Exclusion Criteria: secondary intracerebral hemorrhage resulting from trauma, brain tumor, blood diseases, arteriovenous malformation or aneurysm, etc; patients with severe heart, liver or kidney disease. Intolerance to herbal medicine, patients with allergies patients planning a surgical evacuation of hematoma with severe cerebral hernia at super-early stage patients with poor compliance TCM,Chinese Medicine. AICH, acute intracerebral hemorrhage.CT, computed tomography; GCS, Glasgow coma scale. S2 Table : Full list of Principle investigators and study centers Number of Centre Centre address, zip code Principle Investigator patients NO. randomized Zhangyong Xia, Rui Liaocheng People's Hospital, Liaocheng, 1 39 Zhang, Guangzeng Li Shandong Province, China, 252000 Guangsheng Chen, Boluo County People's Hospital, 2 Bochang Lin, Weiming Huizhou, Guangdong, China, 514610 32 Zhu Qianshan Zhao, Richao Jiangmen Wuyi Traditional Chinese Medicine 3 Chen, Yongtong He Hospital, Jiangmen, Guangdong, China, 9 529000 Jiexia Li, Xiaomei Huang, The hospital of Chinese Medicine of Conghua 4 27 Mengxin Huang City, Conghua, Guangdong, China, 5109000 Chaojun Chen, Jianfang Guangzhou Hospital of Integrated traditional 5 Hu, Peiqun -

CHINESE GLOSSARY an Bai Ju Yi Bao Pu Zi
CHINESE GLOSSARY an Bai Ju Yi Bao Pu Zi - Nei Pian Bao Shu Ya ba yu jiao yang Bei Ji Qian Jin Yao Fang Beijing ben cao Ben Cao Gang Mu Ben Cao Shi Yi bi guan bian tong bie Bo Yi bu yi cang chang sheng bu si Chen Cang Qi Chen Cun Chen Guang Lei Chen Liang Chen Liang Ji Chen Qian Chu Xian Sheng Mu Zhi Ming Chen Que Chen Tian Hua Cheng Cheng-Chung Shu Chu Cheng Hao Cheng I Cheng Shu De Ruiping Fan (ed.), Confucian Bioethics, 285-291. © 1999 Kluwer Academic Publishers. Printed in Great Britain. 286 CHINESE GLOSSARY Cheng Ying Cheng-Zhu Chou Fu Chu Chu Ci Ji Zhu Chuang Tzu (Zhuang Zi) Chuang Tzu (Zhuang Zi) Chun Qiu Fan Lu Chun Qiu Fan Lu -Xun Tian Zhi Dao ci Da Kuang Da Qing Lu Li - Ming Li da ti da tong Da Xue Da Zheng Xin Xiu Da Zang Jing dao (tao) dao bu yuan ren dao jia dao li dao xue dao yi de de xing Diao Qu Yuan Fu dong Dong Zhong Shu Du Si Shu Da Chuan Shuo DuanWu Du Shu Er Ya fan guan CHINESE GLOSSARY 287 Fan Li Sao feng, han, shu, shi, zao, huo Fu Lei fu zuo Fun You-lan Gao Seng Zhuan Ge Hong ge wu, zhi zhi, cheng yi, zheng xin, xiu shen, qi jia, zhi guo, ping tian xia Gong Ting Xian gu dai zhong guo ren de jia zhi guan: Jia zhi qu xiang de chong tu ji qi jie xiao Gu Yan Wu Gu Zhu guan xing Guan Yu Guan Zhong Guan Zi Guan Zi - Nei Ye Pian gui shen Guo Dai Dong Han Han Xue Yan Jiu Zhong Xin Han Yu he He Xian Ming Hua Shan Wen Yi Hua Wen Shu Ju Hua Zhong Li Gong Da Xue Huang Di Nei Jing Su Wen Huang Jun Jie Huang Ru Cheng Huang Zhong Mo 288 CHINESE GLOSSARY Huang Zi Ping Huang Zong Xi jen (ren) Jia Yi Jiao Xun jie cao jie lie jinga -

Moral Psychology and Cultivating the Self
Edinburgh Research Explorer Moral psychology and cultivating the self Citation for published version: Virag, C 2019, Moral psychology and cultivating the self. in PJ Ivanhoe (ed.), Zhu Xi: Selected Writings. Oxford Chinese Thought, Oxford University Press, pp. 35-55. https://doi.org/10.1093/oso/9780190861254.003.0003 Digital Object Identifier (DOI): 10.1093/oso/9780190861254.003.0003 Link: Link to publication record in Edinburgh Research Explorer Document Version: Peer reviewed version Published In: Zhu Xi Publisher Rights Statement: This is a pre-copyedited, author-produced version of a chapter published following peer review. The version of record: Virag, C., 2019, "Moral Psychology and Cultivating the Self", in P. J. Ivanhoe (ed), Zhu Xi: Selected Writings, Oxford University Press, DOI: 10.1093/oso/9780190861254.001.0001, is available online at: https://www.oxfordscholarship.com/view/10.1093/oso/9780190861254.001.0001/oso-9780190861254-chapter-3 General rights Copyright for the publications made accessible via the Edinburgh Research Explorer is retained by the author(s) and / or other copyright owners and it is a condition of accessing these publications that users recognise and abide by the legal requirements associated with these rights. Take down policy The University of Edinburgh has made every reasonable effort to ensure that Edinburgh Research Explorer content complies with UK legislation. If you believe that the public display of this file breaches copyright please contact [email protected] providing details, and we will remove -

Dao Yin (Aka Qigong): Origin, Development, Potential Mechanisms, and Clinical Applications
Hindawi Evidence-Based Complementary and Alternative Medicine Volume 2019, Article ID 3705120, 11 pages https://doi.org/10.1155/2019/3705120 Review Article Dao Yin (a.k.a. Qigong): Origin, Development, Potential Mechanisms, and Clinical Applications Xiaorong Chen ,1 Jiabao Cui,2 Ru Li,1 Richard Norton ,3 Joel Park ,4 Jian Kong,4 and Albert Yeung 3,4 1Faculty of Physical Education, Shenzhen University, Shenzhen 518060, China 2South China Normal University, Guangzhou 510006, China 3Depression Clinical and Research Program, Massachusetts General Hospital, Boston, MA, USA 4Department of Psychiatry, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA Correspondence should be addressed to Albert Yeung; [email protected] Received 25 June 2019; Accepted 13 September 2019; Published 21 October 2019 Academic Editor: Jenny M. Wilkinson Copyright © 2019 Xiaorong Chen et al. *is is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Dao Yin is a form of exercise combining physical movements, mental focus, and breathing originated in ancient China. In this review, we introduce the history in the development and the scope of Dao Yin, the relationship between Dao Yin with Taoist culture and Qigong, and the potential mechanisms of how Dao Yin promotes health and alleviate illnesses. Empirical research studies using Dao Yin for treatment of lumbar spondylosis, peripheral musculoskeletal -

Le Pallet Bei Nantes 1079-1142 Kloster St. Marcel, Saone) : Philosoph Biographie 1928 Ye, Lingfeng
Report Title - p. 1 of 664 Report Title Abélard, Pierre = Abaillard, Pierre = Abaelardus, Petrus (Le Pallet bei Nantes 1079-1142 Kloster St. Marcel, Saone) : Philosoph Biographie 1928 Ye, Lingfeng. Abola yu Ailüqisi de qing shu [ID D14244]. [Advertisement for Lettres d'Héloïse et d'Abailard ; transl. by Liang Shiqiu. In : Xin yue ; vol. 1, no 7 (1928). "This is a love story which happened 800 years ago. A nun and a monk have written a bundle of love letters. No love letters, whether in China or in a foreign country, are more grief-stricken, more sadly touching and more sublime than those found in this volume. The beautiful and ingenious lines have become popular quotations of lovers in later generations, showing the greatness of their influence. The most admirable point is that there is nothing frivolous in these poems, and the translator considers this anthology a 'transcendent and holy' masterpiece." [Babb23] Bibliographie : Autor 1928 [Abélard, Pierre]. Abola yu Ailüqisi de qing shu. Peter Abelard zhu ; Liang Shiqiu yi. In : Xin Yue ; vol. 1, no 8 (Oct. 10 1928). = (Shanghai : Shang wu yin shu guan, 1935). (Shi jie wen xue ming zhu). Übersetzung von Lettres d'Héloïse et d'Abailard. Ed. ornée de huit figures gravées par les meilleurs artistes de Paris, d'après les dessins et sous la direction de Moreau le jeune. Vol. 1-3. (Paris : J.B. Fournier, 1796). [Babb23] Alain = Chartier, Emile = Chartier, Emile-Auguste (Mortagne-sur-Huisne = Mortagne-au-Perche 1868-1951 Le Vésinet) : Philosoph, Schriftsteller, Journalist Bibliographie : Autor 1972 [Alain]. Xing fu lun. Chatai'er zhuan ; Zheng Jieyun zhong yi. -

American Association of Chinese Studies 58Th Annual Conference
American Association of Chinese Studies 58th Annual Conference Program Pepperdine University October 7 – 9, 2016, Malibu, California Hosted by Pepperdine University Malibu, California www.pepperdine.edu 2 Program Overview Hotel: Hampton Inn & Suites Agoura Hills, 30255 Agoura Hills Road, Agoura Hills, CA 91301 Tel: 818-597-0333 www.agourahills.hamptoninn.com Conference Location: Pepperdine University 24255 Pacific Coast Highway, Malibu, CA 90263 Phone: 310-506-4000 Directions from Hampton Inn & Suites to Pepperdine University: Take US-101 S/Ventura Freeway to the exit for Lost Hills Rd. Turn right onto Lost Hills Rd. and then turn right onto Las Virgenes Rd. Continue onto Malibu Canyon Rd. and then turn right onto Seaver Dr. to arrive at Pepperdine University. Shuttle Schedule from Hampton Inn & Suites to Pepperdine University Saturday, Oct. 8 Pick ups at 7:30 and 8:30 a.m. from hotel; please note that everyone cannot necessarily be accommodated in the second bus; some participants should take to first bus to allow for more space on the second. 3 Pick up at 6:15 p.m. at Seaver College main lot for run back to hotel; Pick up at 9:15 p.m. at Villa Graziadio for trip back to hotel. Sunday, Oct. 9 Pick ups at 7:30 and 8:30 a.m. at the hotel for trip to campus; capacity of 30 people per bus, so not everyone should take the latter shuttle; Pick up at 10:00 a.m. at Seaver College for run back to hotel. 4 AACS 2016 Conference: “Engagement and Identity” The 2016 annual meeting of the American Association of Chinese Studies (AACS) is centered on the theme of “Engagement and Identity”, with a variety of academic panels on Chinese politics, economics, humanities and social science. -

Reconstructing the Confucian
Chapter One Zhu Xi, Zhou Dunyi, and the Confucian dao The world into which Zhu Xi was born, as he came to understand it, was in crisis. In 1127, three years before his birth, the capital of Song China, Kaifeng, had been overrun by Jurchen forces from the northeast,1 and both Emperor Qinzong 欽宗 (r. 1126–1127) and the recently abdicated Emperor Huizong 徽宗 (r. 1101–1126) had been abducted. The Song court had fled south and had reestablished itself at the “temporary” capital of Lin’an (modern Hangzhou). The Jurchen, under their Jin dynasty, ruled the northern half of China until 1234, when they in turn were conquered by the Mongols, who proceeded to conquer the rest of China in 1279. So Zhu Xi lived his entire life (1130–1200) with almost half of his homeland under foreign rule, facing the very real threat of a complete loss. These circumstances deeply affected his worldview: China, he felt, needed to reassert itself both militarily and culturally. His father, Zhu Song 朱松 (1097–1143), had been part of the faction that had opposed the appeasement policy followed by Chief Councilor Qin Gui 秦檜 (1090–1155), arguing for more aggressive military action against the Jin,2 and in his earlier career Zhu Xi had argued along the same lines. But 1. The Jurchen (Nuzhen 女真 in Chinese) were the ancestors of the Manchus, who later would overthrow the Ming 明 dynasty (1368–1644) and establish the last imperial Chinese dynasty, the Qing 情 (1644–1911). 2. Chan, “Chu Hsi,” in Franke, Sung Biographies, vol.1:282. -

Foundations of Confucian Thought
FOUNDATIONS OF CONFUCIAN THOUGHT FOUNDATIONS OF CONFUCIAN THOUGHT Intellectual Life in the Chunqiu Period, 722–453 b.c.e. Yuri Pines university of hawai‘i press honolulu © 2002 University of Hawai‘i Press All rights reserved Printed in the United States of America 07 06 05 04 03 02 6 5 4 3 2 1 Library of Congress Cataloging-in-Publication Data Pines, Yuri. Foundations of confucian thought : intellectual life in the Chunqiu period, 722–453 b.c.e. / Yuri Pines. p. cm. Includes bibliographical references and index. ISBN 0-8248-2396-6 (alk. paper) 1. China—Intellectual life—To 221 b.c. 2. China— History—Spring and Autumn period, 722–481. I. Title. DS741.65 .P55 2002 931—dc21 2001046286 University of Hawai‘i Press books are printed on acid-free paper and meet the guidelines for permanence and durability of the Council on Library Resources. Designed by Integrated Composition Systems Printed by The Maple-Vail Book Manufacturing Group Contents Acknowledgments vii Notes on Translation, Terms, and Quotations ix Introduction 1 Chapter 1. Sources of Chunqiu Thought 13 Chapter 2. Heaven and Man Part Ways: Changing Attitudes Toward Divine Authority 55 Chapter 3. The Universal Panacea: Ritual and Preserving Hierarchical Order 89 Chapter 4. The World Falls Apart: A Futile Search for International Order 105 Chapter 5. When a Minister Mounts the Ruler: Chunqiu Views of Loyalty 136 Chapter 6. Nobility of Blood and Spirit: Chunqiu Ethical Thought 164 Chapter 7. The Chunqiu Legacy 205 Appendix 1: Grammatical Change in the Zuo: Case Studies of the “Yu” and “Qi” Particles 217 Appendix 2: Zhanguo Data in the Zuo 221 Appendix 3: Comparing Scribal Accounts in the Zuo 227 Appendix 4: Spurious Speeches and Interpolations in the Zuo 233 Notes 247 List of Chunqiu Personalities 309 Glossary 333 Bibliography 343 Index 373 Acknowledgments This book has developed from my Ph.D. -

The Culture of the Confucianists Is Essential to the Chinese Traditional Civilization, Zhang Jian's the Major Feature
The Influence of Confucianism on Molding Zhang Jian’s Cultural Character Nangtong University Wang Dunqin Summary: Confucianism is the core of the Chinese traditional culture. The major features of Zhang Jian’s cultural character are “Zhong”, “Xin”, “Du”, “Jing”, “Ren”, “Yi”, “Li”, “Jian”, “Ren”, “Shen”. Having read a lot of Confucian classics during his years of imperial examinations, Zhang Jian was deeply influenced by the essence of Chinese traditional culture, the traditional thought pattern, the Confucian doctrines and the orthodox ethics in his world outlook, philosophy of life and values. Key words: Confucianism, Zhang Jian, Cultural Character “Culture” is a popular word with its frequent use, multiple explanations and high controversies. Generally speaking, “culture” may be explained in broad and narrow sense. In broad sense, it can be 1 understood everything that human being creates. In narrow sense, it is a meaning mode that hands down from one generation to another, and it embodies traditional conception in symbol form. Chinese traditional culture is a great one that comes from the long-term social life, and that is orientated by the dominators and aimed to instill into people. For hundreds of thousands of years, the influence of Chinese culture is so strong that it cannot be effaced from people’s mind. Confucianism is the core in the Chinese traditional culture. In a certain sense, the Chinese traditional culture is Confucian culture. Zhang Jian’s cultural character was deeply influenced by Confucianism. Zhang Jian (1853-1926) was an illustrious person in the late Qing dynasty and early Republic of China. He was active on the stage of politics, economy and culture in China, and made grand 2 contribution to modern China. -

Natural Law and Normativity in the Huangdi Sijing
CORE Metadata, citation and similar papers at core.ac.uk Provided by ScholarBank@NUS NATURAL LAW AND NORMATIVITY IN THE HUANGDI SIJING ERNEST KAM CHUEN HWEE (B.A. (Hons), NUS) A THESIS SUBMITTED FOR THE DEGREE OF MASTER OF ARTS DEPARTMENT OF PHILOSOPHY NATIONAL UNIVERSITY OF SINGAPORE 2005 Acknowledgements My interest in Huang-Lao began towards the end of my Honors Year and I acknowledge my debt to Prof. Alan Chan, my supervisor, for introducing me to the Huangdi Sijing as a choice for research. Without his encouragement, I may never have thought of furthering my studies beyond my Bachelor’s Degree. Prof. Chan has also been extremely generous in the advice and support he has given me these past three years. For these and also for painstakingly going through the drafts of this dissertation, I am eternally grateful to him. A special word of thanks is due to A/P Tan Sor Hoon. Though she was not my supervisor, she has been extremely patient in answering whatever queries I have had and tolerant of my unexpected and frequent visits all this while. I am grateful too to all who have helped me during my Graduate Seminar Presentation: Kim Hak Ze for agreeing to lend me his laptop (which the projector unfortunately refused to cooperate with), A/P Nuyen Anh Tuan for volunteering to lend me his, and Weng Hong whose Mac I borrowed eventually. A word of thanks also to all my fellow graduate students and A/P Saranindranath Tagore for their comments and suggestions during the seminar. Outside of NUS, I am thankful to the following professors for their invaluable assistance: Prof. -

Tranquil Sitting: a Taoist Journal on Meditation and Chinese Medical Qigong
“Master Yin Shi Zi’s book so enthralled me that I read it in a single sitting. His training in classical Chinese medicine and as a professor of physiology enables him to express both his own experiences and his guide to cultivating a practice of these methods in a language easily comprehensible to the modern reader. His book is a wonderful contribution to our understanding of the nature of Taoist/Buddhist yoga, meditation, and inner science.” —Glenn H. Mullin, author of Selected Works of the Dalai Lama and Death and Dying “The reader can really better understand the mental and physical phenomena encountered when progressing through meditation. If anyone ever wondered what changes may occur during intense study of meditation, this book helps to provide answers.” —Master Tsung Hwa Jou, author of The Dao of Taijiquan and The Tao of Meditation “This wonderful book has been very influential in my own practice and I was elated to find that Shifu Hwang and Cheney Crow have completed such a clear translation. Tranquil Sitting provides inspiration for all those who want to practice meditation, but may feel that their life contradicts or obstructs that practice. Yin Shi Zi is deservedly considered one of China’s most celebrated meditation practitioners.” —Stuart Alve Olson, author of Cultivating the Ch’i Tranquil Sitting A Taoist Journal on Meditation and Chinese Medical Qigong Yin Shi Zi Translated by Shifu Hwang and Cheney Crow, Ph.D. Forewords by Master Zhongxian Wu and Glenn H. Mullin London and Philadelphia This edition published in 2013 by Singing Dragon an imprint of Jessica Kingsley Publishers 116 Pentonville Road London N1 9JB, UK and 400 Market Street, Suite 400 Philadelphia, PA 19106, USA www.singingdragon.com First published in 1994 by Dragon Door Publications Copyright © Shifu Hwang and Cheney Crow 1994, 2013 Foreword copyright © Master Zhongxian Wu 2013 Foreword copyright © Glenn H.