Understanding Declines in the Population Size of Migratory Birds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Mariinsky Ballet & Orchestra
Thursday, October 1, 2015, 8pm Friday, October 2, 2015, 8pm Saturday, October 3, 2015, 2pm & 8pm Sunday, October 4, 2015, 3pm Zellerbach Hall The Mariinsky Ballet & Orchestra Gavriel Heine, Conductor The Company Diana Vishneva, Nadezhda Batoeva, Anastasia Matvienko, Sofia Gumerova, Ekaterina Chebykina, Kristina Shapran, Elena Bazhenova Vladimir Shklyarov, Konstantin Zverev, Yury Smekalov, Filipp Stepin, Islom Baimuradov, Andrey Yakovlev, Soslan Kulaev, Dmitry Pukhachov Alexandra Somova, Ekaterina Ivannikova, Tamara Gimadieva, Sofia Ivanova-Soblikova, Irina Prokofieva, Anastasia Zaklinskaya, Yuliana Chereshkevich, Lubov Kozharskaya, Yulia Kobzar, Viktoria Brileva, Alisa Krasovskaya, Marina Teterina, Darina Zarubskaya, Olga Gromova, Margarita Frolova, Anna Tolmacheva, Anastasiya Sogrina, Yana Yaschenko, Maria Lebedeva, Alisa Petrenko, Elizaveta Antonova, Alisa Boyarko, Daria Ustyuzhanina, Alexandra Dementieva, Olga Belik, Anastasia Petushkova, Anastasia Mikheikina, Olga Minina, Ksenia Tagunova, Yana Tikhonova, Elena Androsova, Svetlana Ivanova, Ksenia Dubrovina, Ksenia Ostreikovskaya, Diana Smirnova, Renata Shakirova, Alisa Rusina, Ekaterina Krasyuk, Svetlana Russkikh, Irina Tolchilschikova Alexey Popov, Maxim Petrov, Roman Belyakov, Vasily Tkachenko, Andrey Soloviev, Konstantin Ivkin, Alexander Beloborodov, Viktor Litvinenko, Andrey Arseniev, Alexey Atamanov, Nail Enikeev, Vitaly Amelishko, Nikita Lyaschenko, Daniil Lopatin, Yaroslav Baibordin, Evgeny Konovalov, Dmitry Sharapov, Vadim Belyaev, Oleg Demchenko, Alexey Kuzmin, Anatoly Marchenko, -

International Research and Exchanges Board Records
International Research and Exchanges Board Records A Finding Aid to the Collection in the Library of Congress Prepared by Karen Linn Femia, Michael McElderry, and Karen Stuart with the assistance of Jeffery Bryson, Brian McGuire, Jewel McPherson, and Chanté Wilson-Flowers Manuscript Division Library of Congress Washington, D.C. 2011 International Research and Exchanges Board Records Page ii Collection Summary Title: International Research and Exchanges Board Records Span Dates: 1947-1991 (bulk 1956-1983) ID No: MSS80702 Creator: International Research and Exchanges Board Creator: Inter-University Committee on Travel Grants Extent: 331,000 items; 331 cartons; 397.2 linear feet Language: Collection material in English and Russian Repository: Manuscript Division, Library of Congress, Washington, D.C. Abstract: American service organization sponsoring scholarly exchange programs with the Soviet Union and Eastern Europe in the Cold War era. Correspondence, case files, subject files, reports, financial records, printed matter, and other records documenting participants’ personal experiences and research projects as well as the administrative operations, selection process, and collaborative projects of one of America’s principal academic exchange programs. International Research and Exchanges Board Records Page iii Contents Collection Summary .......................................................... ii Administrative Information ......................................................1 Organizational History..........................................................2 -

Papers in Pidgin and Creole Linguistics No. 5
Papers in pidgin and creole linguistics No.5 Mühlhäusler, P. editor. Papers in Pidgin and Creole Linguistics No. 5. A-91, v + 213 pages. Pacific Linguistics, The Australian National University, 1998. DOI:10.15144/PL-A91.cover ©1998 Pacific Linguistics and/or the author(s). Online edition licensed 2015 CC BY-SA 4.0, with permission of PL. A sealang.net/CRCL initiative. PACIFIC LINGUISTICS FOUNDING EDITOR: Stephen A. Wurrn EDITORIAL BOARD: Malcolm D. Ross and Darrell T. Tryon (Managing Editors), Thomas E. Dutton, Nikolaus P. Himmelmann, Andrew K. Pawley Pacific Linguistics is a publisher specialising in linguistic descriptions, dictionaries, atlases and other material on languages of the Pacific, the Philippines, Indonesia and Southeast Asia. The authors and editors of Pacific Linguistics publications are drawn from a wide range of institutions around the world. Pacific Linguistics is associated with the Research School of Pacific and Asian Studies at the Australian National University. Pacific Linguistics was established in 1963 through an initial grant from the Hunter Douglas Fund. It is a non-profit-making body financedlargely from the sales of its books to libraries and individuals throughout the world, with some assistance from the School. The Editorial Board of Pacific Linguistics is made up of the academic staff of the School's Department of Linguistics. The Board also appoints a body of editorial advisors drawn from the international community of linguists. Publications in Series A, B and C and textbooks in Series D are refereed by scholars with relevant expertise who are normally not members of the editorial board. To date Pacific Linguistics has published over 400 volumes in four series: -Series A: Occasional Papers; collections of shorter papers, usually on a single topic or area. -

Long-Term Outcomes Resulting from Exposure to Stress and Fluoxetine During Development
University of Calgary PRISM: University of Calgary's Digital Repository Graduate Studies The Vault: Electronic Theses and Dissertations 2017 Long-Term Outcomes Resulting from Exposure to Stress and Fluoxetine During Development Kiryanova, Veronika Kiryanova, V. (2017). Long-Term Outcomes Resulting from Exposure to Stress and Fluoxetine During Development (Unpublished doctoral thesis). University of Calgary, Calgary, AB. doi:10.11575/PRISM/26786 http://hdl.handle.net/11023/3769 doctoral thesis University of Calgary graduate students retain copyright ownership and moral rights for their thesis. You may use this material in any way that is permitted by the Copyright Act or through licensing that has been assigned to the document. For uses that are not allowable under copyright legislation or licensing, you are required to seek permission. Downloaded from PRISM: https://prism.ucalgary.ca UNIVERSITY OF CALGARY Long-Term Outcomes Resulting from Exposure to Stress and Fluoxetine During Development by Veronika Kiryanova A THESIS SUBMITTED TO THE FACULTY OF GRADUATE STUDIES IN PARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY GRADUATE PROGRAM IN PSYCHOLOGY CALGARY, ALBERTA APRIL, 2017 © Veronika Kiryanova 2017 1 Abstract Depression, anxiety, and stress are common in pregnant women. One of the primary pharmacological treatments for anxiety and depression is the antidepressant fluoxetine (Flx). Maternal stress, depression, and Flx exposure are known to effect neurodevelopment of the offspring; however, their combined effects have been scarcely studied. In this thesis, we examine the combined effects of maternal stress during pregnancy and perinatal exposure to Flx on the behaviour and the biochemical outcomes of mice as adults. -

Czech Music in Nebraska
Czech Music in Nebraska Ceska Hudba v Nebrasce OUR COVER PICTURE The Pavlik Band of Verdigre, Nebraska, was organized in 1878 by the five Pavlik brothers: Matej, John, Albert, Charles and Vaclav. Mr. Vaclav Tomek also played in the band. (Photo courtesy of Edward S. Pavlik, Verdigre, Neraska). Editor Vladimir Kucera Co-editor DeLores Kucera Copyright 1980 by Vladimir Kucera DeLores Kucera Published 1980 Bohemians (Czechs) as a whole are extremely fond of dramatic performances. One of their sayings is “The stage is the school of life.” A very large percentage are good musicians, so that wherever even a small group lives, they are sure to have a very good band. Ruzena Rosicka They love their native music, with its pronounced and unusual rhythm especially when played by their somewhat martial bands. A Guide to the Cornhusker State Czechs—A Nation of Musicians An importantCzechoslovakian folklore is music. Song and music at all times used to accompany man from the cradle to the grave and were a necessary accompaniment of all important family events. The most popular of the musical instruments were bagpipes, usually with violin, clarinet and cembalo accompaniment. Typical for pastoral soloist music were different types of fifes and horns, the latter often monstrous contraptions, several feet long. Traditional folk music has been at present superseded by modern forms, but old rural musical instruments and popular tunes have been revived in amateur groups of folklore music or during folklore festivals. ZLATE CESKE VZPOMINKY GOLDEN CZECH MEMORIES There is an old proverb which says that every Czech is born, not with a silver spoon in his mouth, but with a violin under his pillow. -

2019 Unclaimed Property Report
NOTICE TO OWNERS OF ABANDONED PROPERTY: 2019 UNCLAIMED PROPERTY REPORT State Treasurer John Murante 402-471-8497 | 877-572-9688 treasurer.nebraska.gov Unclaimed Property Division 809 P Street Lincoln, NE 68508 Dear Nebraskans, KUHLMANN ORTHODONTICS STEINSLAND VICKI A WITT TOM W KRAMER TODD WINTERS CORY J HART KENNETH R MOORE DEBRA S SWANSON MATHEW CLAIM TO STATE OF NEBRASKA FOR UNCLAIMED PROPERTY Reminder: Information concerning the GAYLE Y PERSHING STEMMERMAN WOLFE BRIAN LOWE JACK YOUNG PATRICK R HENDRICKSON MOORE KEVIN SZENASI CYLVIA KUNSELMAN ADA E PAINE DONNA CATHERNE COLIN E F MR. Thank you for your interest in the 2019 Property ID Number(s) (if known): How did you become aware of this property? WOODWARD MCCASLAND TAYLORHERDT LIZ “Claimant” means person claiming property. amount or description of the property and LARA JOSE JR PALACIOS AUCIN STORMS DAKOTA R DANNY VIRGILENE HENDRICKSON MULHERN LINDA J THOMAS BURDETTE Unclaimed Property Newspaper Publication BOX BUTTE Unclaimed Property Report. Unclaimed “Owner” means name as listed with the State Treasurer. LE VU A WILMER DAVID STORY LINDA WURDEMAN SARAH N MUNGER TIMOTHY TOMS AUTO & CYCLE Nebraska State Fair the name and address of the holder may PARR MADELINE TIFFANY ADAMS MICHAEL HENZLER DEBRA J property can come in many different Husker Harvest Days LEFFLER ROBERT STRATEGIC PIONEER BANNER MUNRO ALLEN W REPAIR Claimant’s Name and Present Address: Claimant is: LEMIRAND PATTNO TOM J STREFF BRIAN WYMORE ERMA M BAKKEHAUG HENZLER RONALD L MURPHY SHIRLEY M TOOLEY MICHAEL J Other Outreach -

Music by Pyotr Tchaikovsky Mariinsky Ballet Musical Direction by Valery
BAM 2015 Winter/Spring Season #MariinskyBAM Brooklyn Academy of Music The Mariinsky Theatre Alan H. Fishman, Valery Gergiev, Chairman of the Board General and Artistic Director, State Academic Mariinsky Theatre William I. Campbell, Frederick Iseman, Vice Chairman of the Board Chairman, Mariinsky Foundation of America Adam E. Max, Donald M. Kendall, Vice Chairman of the Board Chairman Emeritus Karen Brooks Hopkins, Michael D. White, President Vice Chairman Joseph V. Melillo, Executive Producer SwanBAM and the Mariinsky Theatre present Lake Season Sponsor: Music by Pyotr Tchaikovsky Mariinsky Ballet Musical direction by Valery Gergiev BAM engagement made possible by: BAM Howard Gilman Opera House Jan 15 at 7pm (conducted by Valery Gergiev); Jan 16 & 21—23 at 7:30pm Leadership support for the Mariinsky Residency at Running time: three hours and 10 minutes, BAM provided by Frederick Iseman including two intermissions VTB Bank is the Principal Partner of the Mariinsky Theatre. Sberbank and Yoko Nagae Ceschina are Libretto by Vladimir Begichev and Vasily Geltzer Principal Sponsors. Choreography by Marius Petipa and Lev Ivanov (1895) Revised choreography and stage direction by Support for the Signature Artists Series provided by the Howard Gilman Foundation Konstantin Sergeyev (1950) Set design by Igor Ivanov Major support for the Mariinsky Residency at BAM provided by Renova Costume design by Galina Solovyova Leadership support: The Harkness Foundation for Dance The SHS Foundation Swan Lake—Casting Odette-Odile Viktoria Tereshkina (1/15, 1/23) -
First Instrument Middle School Band Alphabetical Order
First Instrument Middle School Band Alphabetical Order Laurence Elisha Browning Alto Saxophone Melody Chaclan Alto Saxophone Eric Kwon Alto Saxophone Zachary R. Lin Alto Saxophone Emma Grace Mina Maldo Alto Saxophone Surya Subbarao Alto Saxophone Justin Tsai Alto Saxophone Andrew Vitt Alto Saxophone Isaac Stephen Chung Baritone Saxophone Moses Moon Baritone Saxophone Zion Abendano Baritone/Euphonium Jacob San Jose Baritone/Euphonium Esmeralda Lagunas Baritone/Euphonium Elton Manchester Baritone/Euphonium Ava Martin Baritone/Euphonium Erin Miyahara Baritone/Euphonium Jasmine Chen Bass Clarinet Vanessa Chen Bass Clarinet Anika Cortes Bass Clarinet Joseph Wangsa Bass Clarinet Sachin Bhajekar Bassoon Christopher Del Poso Bassoon Anson Fung Bassoon Vishnu Manathattai Bassoon Shaun Baek Bb Clarinet Sunny Cai Bb Clarinet Michelle Cheng Bb Clarinet Jaemin Choi Bb Clarinet Andrew Wooyong Chung Bb Clarinet Dylan Davidoff Bb Clarinet Aaron Agustin Huerta Bb Clarinet Kaelan James Bb Clarinet Andrew Kang Bb Clarinet Jason Kim Bb Clarinet Sean Kim Bb Clarinet Daniel Lee Bb Clarinet Dustin Lee Bb Clarinet Isaac Ng Bb Clarinet Xixi Richards Bb Clarinet Alexandra Schoettler Bb Clarinet Emily Shanmugam Bb Clarinet Carson Tanner Bb Clarinet Christopher Uy Bb Clarinet Bing Wi Bb Clarinet Andrew Wu Bb Clarinet Rachel Yang Bb Clarinet Isaac Yoon Bb Clarinet Cooper Zee Bb Clarinet Rongyu Zhang Bb Clarinet Nithin Chilakapati * Flute Alexis Cruz Flute Cassie Kao Flute Annie Lee Flute Dayoon Lee Flute Joanne Lee Flute Vivian Lee Flute Alissa Shterenberg Flute Aileen -
Jackson Appeals Suspension from Proctor Holt When Police Officers Returned, Safety Or Medical Or Psychological Jackson's Punishment Too Harsh
Volume 75, Number 24 NewsWRESHE is the plural of new. Friday, MarcRh 25,1988 Jackson appeals suspension from Proctor Holt When police officers returned, safety or medical or psychological Jackson's punishment too harsh. nurturing community, but then here's Jackson again verbally abused welfare of any member of the univer- After being notified by the proctor Holt saying, 'See you later, buddy," Veitman. Veitman handcuffed sity." In such cases, the proctor must of the proctor's taking independent says Menendez. Jackson and placed him under arrest. "inform and act with the concurrence action in the case, Norman asked Holt Menendez says that Jackson has "It was my own fault," Jackson of the Chairman of the University to consider Jackson's financial and contributed to the Rice community as says. "I'm not saying I'm innocent." Review Board after consultation with political situation. He has since spo- an S.A. senator, a rugby player, a According to Jackson, the police the Master, an appropriate represen- ken with both Holt and Ronald Steb- freshman advisor, and in his work for report states, '"Jackson was disor- tative of the College government and bings, Vice President for Student the Economics Department derly for using vulgar language in the chairman of the University court Affairs, on Jackson's behalf. Jackson had planned to run for public.'" [sic]." Norman says that Stebbings rec- Lovett social coordinator, in conjunc- "There was no destruction of uni- Holt says that though he called the ommended that Jackson write Holt a tion with three other Lovett members, versity property," Jackson says. -
First Name Last Name Country Jeffrey Honey CA Joseph
First_Name Last_Name Country Jeffrey Honey CA Joseph Maceyovski CA Marianne Steenken DE Rachel Wolf US Kent Minault US Tom Walsh US David Rangel US John Morrison US Frances Goff US Douglas Estes US Robert Gore US David Ellringer US Ronald Gelden US Clifford Johnson US Kathleen Maher US Simone Morgen US Patrick De La Garza Und Senkel US Deborah Richmond CA Bertha Kriegler US Linda Owen US Vincent De Stefano US Gordon Edwards AU Debra Rehn US Rik Reynolds US David Greene US mathew thomas AU chris Helcermanas-Benge CA john papandrea US Warren Kazor CA glenn kitashima US Eric Stordahl US Cynthia Curtis US Meryle A. Korn US DBellUS Ovina Feldman US Nancy Wrensch US Michael Dennis US Frank Heacock US Kathy Spera US Ryan Moore US Hartson Doak US Kerry Weaver US Larry Blount US Thomas Windberg US susan peirce US Ian Carman US Mark Jacobs US Karen Bowden US Lisa Butterfield US Becky Daiss US Tom Winstead US Joe Ferriter US Lori Mulvey US Anita Chariw US Donald Cronin US Ernest Montoro US RICHARD CURRY US Stephen and Robin Newberg US Michael David Sasson US James Milnthorpe US Ann Thryft US Allie Robbins US Terrie Williams US Michael Tomczyszyn US Mark Gallegos US John Hendricks US John Keiser US Ron Blankenship US Susan Osada US Jim Steitz US Ron Schutte US John Bernard US JOSEPH LITE US Guy Zahller US Jim Newton US Liam Black CA William Lee Kohler US Allan Campbell US Mark Cappetta US Cornelius Coughlin US Dee Randolph US Anthony Trupiano US Glenn Martin US Robert Justice US Ken Kolbe US Joseph Kelley US dale riehart US Jeffrey Hearn US Shawn Dufraine US Brent Rocks US CHARLES HANCOCK US John Holmes US Terrence Parkhurst US Douglas Buckley US Sister Carol Boschert US Michael Landers US Frank Hyden US Randall Paske US Janice Foss US Wojciech Rowinski US Robert Ziegler US David Dresser US Donna Bonetti US Bob Thomas US Robert Angone US Patty Bonney US Joe Rogers US Kathryn Rose US John Bill US Vincent O'Neill US William Hulme US Leonard Tremmel US Janet Martin CA Callie Riley US Galloway Allbright US Michael Klausing US Christine Roane US Gary Grice US The Rev. -
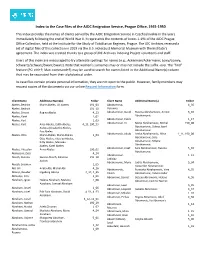
Index to the Case Files of the AJDC Emigration Service, Prague Office, 1945-1950
Index to the Case Files of the AJDC Emigration Service, Prague Office, 1945-1950 This index provides the names of clients served by the AJDC Emigration Service in Czechoslovakia in the years immediately following the end of World War II. It represents the contents of boxes 1-191 of the AJDC Prague Office Collection, held at the Institute for the Study of Totalitarian Regimes, Prague. The JDC Archives received a set of digital files of this collection in 2019 via the U.S. Holocaust Memorial Museum with the Institute’s agreement. The index was created thanks to a group of JDC Archives Indexing Project volunteers and staff. Users of this index are encouraged to try alternate spellings for names (e.g., Ackerman/Ackermann; Lowy/Loewy; Schwartz/Schwarz/Swarc/Swarz). Note that women’s surnames may or may not include the suffix -ova. The “find” feature (PC: ctrl+F; Mac: command+F) may be used to search for names listed in the Additional Name(s) column that may be separated from their alphabetical order. As case files contain private personal information, they are not open to the public. However, family members may request copies of the documents via our online Request Information form. Client Name Additional Name(s) Folder Client Name Additional Name(s) Folder Abeles, Bedrich Marie Abeles, Jiri Abeles 191_04 Abrahamova, 4_56 Abeles, Bedrich 191_10 Veronika Abrahamovic, David Ruzena Abrahamovic, Arnost 5_02 Abeles, Ernest Regina Abeles 4_13 Abrahamovic Abeles, Karel 1_02 Abeles, Kurt 1_03 Abrahamovic, Evzen 1_17 Abeles, Kurt Alice Abeles, Edith -

PERSONS SENTENCED to DEATH in CANADA, 1867-1976: An
1 PERSONS SENTENCED TO DEATH IN CANADA, 1867-1976: An Inventory of Case Files in the Fonds of the Department of Justice Lorraine GADOURY and Antonio LECHASSEUR Government Archives Division National Archives of Canada 2 This reference tool is intended for research use. It may be reproduced, except for sale or advertising purposes, provided credit is given to the National Archives of Canada, and to the authors. Access to records described in this reference tool is governed by the provisions of the Access to Information and Privacy Acts and regulations made pursuant to the legislation. Cet ouvrage de référence est disponible en français. 8 National Archives of Canada, 1994 3 TABLE OF CONTENTS Table of Contents.............................................................................................................................3 Abbreviations...................................................................................................................................4 Introduction......................................................................................................................................5 Index of sentences by year...............................................................................................................7 Index of sentences by province......................................................................................................11 Index of the victims .......................................................................................................................13 Index