Advances in Passive Sampling: Measuring Chemical Transport and Human Exposures
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Environmental Properties of Chemicals Volume 2
1 t ENVIRONMENTAL 1 PROTECTION Esa Nikunen . Riitta Leinonen Birgit Kemiläinen • Arto Kultamaa Environmental properties of chemicals Volume 2 1 O O O O O O O O OO O OOOOOO Ol OIOOO FINNISH ENVIRONMENT INSTITUTE • EDITA Esa Nikunen e Riitta Leinonen Birgit Kemiläinen • Arto Kultamaa Environmental properties of chemicals Volume 2 HELSINKI 1000 OlO 00000001 00000000000000000 Th/s is a second revfsed version of Environmental Properties of Chemica/s, published by VAPK-Pub/ishing and Ministry of Environment, Environmental Protection Department as Research Report 91, 1990. The pubiication is also available as a CD ROM version: EnviChem 2.0, a PC database runniny under Windows operating systems. ISBN 951-7-2967-2 (publisher) ISBN 952-7 1-0670-0 (co-publisher) ISSN 1238-8602 Layout: Pikseri Julkaisupalvelut Cover illustration: Jussi Hirvi Edita Ltd. Helsinki 2000 Environmental properties of chemicals Volume 2 _____ _____________________________________________________ Contents . VOLUME ONE 1 Contents of the report 2 Environmental properties of chemicals 3 Abbreviations and explanations 7 3.1 Ways of exposure 7 3.2 Exposed species 7 3.3 Fffects________________________________ 7 3.4 Length of exposure 7 3.5 Odour thresholds 8 3.6 Toxicity endpoints 9 3.7 Other abbreviations 9 4 Listofexposedspecies 10 4.1 Mammais 10 4.2 Plants 13 4.3 Birds 14 4.4 Insects 17 4.5 Fishes 1$ 4.6 Mollusca 22 4.7 Crustaceans 23 4.8 Algae 24 4.9 Others 25 5 References 27 Index 1 List of chemicals in alphabetical order - 169 Index II List of chemicals in CAS-number order -

Development of Microplate Screening System
DEVELOPMENT OF MICROPLATE SCREENING SYSTEM AND CONSTRUCTION OF FLUORESCEIN-BASED LIBRARY FENG SUIHAN A THESIS SUMBITTED FOR THE DEGREE OF MASTER OF SCIENCE DEPARTMENT OF CHEMISTRY NATIONAL UNIVERSITY OF SINGAPORE 2010 Acknowledgements First of all, I would like to express my deep gratitude to my supervisor, Associate Professor Young-Tae Chang for his profound knowledge, invaluable guidance, constant support and inspiration throughout my graduate studies. The knowledge, both scientific and otherwise, that I accumulated under his supervision, will aid me greatly throughout my life. Next, I would like to give my sincere thanks to Dr. Marc Vendrell and Dr. Hyung-Ho Ha, for their warm support during my entire graduate studies. Besides, my sincere thinks goes to Miss Siqiang Yang, who helped me to set up the microplate screening platform. Also, I would like to thank all the graduate students of Chang lab, Animesh, Duanting, Ghosh, Jun-Seok, Yun-Kyung, Raj, for their cordiality and friendship. We had a great time together. I also benefited from all other lab members, particularly but not limited to, Siti, Yee Ling, Chew Yan, Xubin, Jeffrey, Shin Hui, Dr. Bi, Dr. Cho, Dr. Jun Li, Dr. Lees, Dr. Kang, Dr. Kim, Dr. Park, and etc. Thanks for making the working place an enjoyable one. I am grateful to Dr. Tan for recruiting me into NUS Medicinal Chemistry graduate program and I am also thankful to NUS for awarding me the research scholarship. At last, I would like to express greatest thanks to my family, particularly my wife, Bai Yang, whose encourage and understanding help me to finish my graduate study in NUS. -

(12) United States Patent (10) Patent No.: US 6,528,079 B2 Podszun Et Al
US006528079B2 (12) United States Patent (10) Patent No.: US 6,528,079 B2 PodsZun et al. (45) Date of Patent: *Mar. 4, 2003 (54) SHAPED BODIES WHICH RELEASE 4,845,106 A 7/1989 Shiokawa et al. .......... 514/342 AGROCHEMICALACTIVE SUBSTANCES 4,849,432 A 7/1989 Shiokawa et al. .......... 514/341 4.882,344 A 11/1989 Shiokawa et al. .......... 514/342 (75) Inventors: Wolfgang Podszun, Köln (DE); Uwe 4.914,113 A 4/1990 Shiokawa et al. .......... 514/333 Priesnitz, Solingen (DE); Jirgen 4,918,086 A 4/1990 Gsell .......................... 514/351 4,918,088 A 4/1990 Gsell .......................... 514/357 Hölters, Leverkusen (DE); Bodo 4.948.798.2- Y - A 8/1990 Gsell .......................... 514/275 Rehbold, Köln (DE); Rafel Israels, 4,963,572 A 10/1990 Gsell .......................... 514/357 Monheim (DE) 4,963,574. A 10/1990 Bachmann et al. ......... 514/357 4,988,712 A 1/1991 Shiokawa et al. .......... 514/340 (73) Assignee: Bayer Aktiengesellschaft, Leverkusen 5,001,138 A 3/1991 Shiokawa et al. .......... 514/342 (DE) 5,034.404 A 7/1991. Uneme et al. .............. 514/365 5,034,524 A 7/1991 Shiokawa et al. .......... 544/124 (*) Notice: This patent issued on a continued pros- 5,039,686 A 8/1991 Davies et al. ............... 514/341 ecution application filed under 37 CFR 5,049,571. A 9/1991 Gsell .......................... 514/345 1.53(d), and is subject to the twenty year SE A :1: S. - - - - - - - -tal.5 E.s pass" provisions of 35 U.S.C. 5,166,1642Y- - - 2 A 11/1992 NanjoOKaWa et al. -

Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2019 Theinternational Programme on Chemical Safety (IPCS) Was Established in 1980
The WHO Recommended Classi cation of Pesticides by Hazard and Guidelines to Classi cation 2019 cation Hazard of Pesticides by and Guidelines to Classi The WHO Recommended Classi The WHO Recommended Classi cation of Pesticides by Hazard and Guidelines to Classi cation 2019 The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2019 TheInternational Programme on Chemical Safety (IPCS) was established in 1980. The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management of chemicals. This publication was developed in the IOMC context. The contents do not necessarily reflect the views or stated policies of individual IOMC Participating Organizations. The Inter-Organization Programme for the Sound Management of Chemicals (IOMC) was established in 1995 following recommendations made by the 1992 UN Conference on Environment and Development to strengthen cooperation and increase international coordination in the field of chemical safety. The Participating Organizations are: FAO, ILO, UNDP, UNEP, UNIDO, UNITAR, WHO, World Bank and OECD. The purpose of the IOMC is to promote coordination of the policies and activities pursued by the Participating Organizations, jointly or separately, to achieve the sound management of chemicals in relation to human health and the environment. WHO recommended classification of pesticides by hazard and guidelines to classification, 2019 edition ISBN 978-92-4-000566-2 (electronic version) ISBN 978-92-4-000567-9 (print version) ISSN 1684-1042 © World Health Organization 2020 Some rights reserved. -

Drug-Like Properties and ADME of Xanthone Derivatives: the Antechamber of Clinical Trials Ana Sara Gomes, Pedro Brandão, Carla
Drug-like properties and ADME of xanthone derivatives: the antechamber of clinical trials Ana Sara Gomes, Pedro Brandão, Carla Fernandes, Marta Correia-da-Silva, Emília Sousa*, Madalena Pinto# # all authors contributed equally to this work *corresponding author Abstract Xanthone derivatives have been described as compounds with privileged scaffolds that exhibited diverse interesting biological activities, such as antitumor activity, directing the interest to pursue the development of these derivatives into drug candidates. Nevertheless, to achieve this purpose it is crucial to study their pharmacokinetics and toxicity (PK/tox) as decision endpoints to continue or interrupt the development investment. This review aims to expose the most relevant analytical methods used in physicochemical and PK/tox studies in order to detect, quantify and identify different bioactive xanthones. Also the methodologies used in the mentioned studies, and the main obtained results, are referred to understand the drugability of xanthones derivatives through in vitro and in vivo systems towards ADME/tox properties, such as physicochemical and metabolic stability and biovailability. The last section of this review focus on a case-study of the development of the drug candidate DMXAA, which has reached clinical trials, to understand the paths and the importance of PK/tox studies. In the end, the data assembled in this review intends to facilitate the design of potential drug candidates with a xanthonic scaffold. Xanthone; analytical method; drug-like; pharmacokinetics; toxicity; drug development; preclinical; metabolism; chromatography. 1. Introduction The xanthone nucleus or 9H-xanthen-9-one (dibenzo-γ-pirone 1, Fig. 1) comprises an important class of oxygenated heterocycles and is considered a privileged structure 1. -

IMDG Code (Amendment 37-14) Index Korean Register of Shipping
IMDG Code (Amendment 37-14) Index Substance, material or article MP Class UN No. ACETAL - 3 1088 ACETALDEHYDE - 3 1089 ACETALDEHYDE AMMONIA - 9 1841 Acetaldehyde diethyl acetal, see - 3 1088 ACETALDEHYDE OXIME - 3 2332 Acetaldol, see - 6.1 2839 beta-Acetaldoxime, see - 3 2332 ACETIC ACID, GLACIAL - 8 2789 ACETIC ACID SOLUTION more than 10% and less than 50% acid, - 8 2790 by mass ACETIC ACID SOLUTION not less than 50% but no more than 80% - 8 2790 acid, by mass ACETIC ACID SOLUTION more than 80% acid, by mass - 8 2789 Acetic aldehyde, see - 3 1089 ACETIC ANHYDRIDE - 8 1715 Acetic oxide, see - 8 1715 Acetoin, see - 3 2621 ACETONE - 3 1090 ACETONE CYANOHYDRIN, STABILIZED P 6.1 1541 Acetone hexafluoride, see - 2.3 2420 ACETONE OILS - 3 1091 Acetone-pyrogallol copolymer 2-diazo-1-naphthol-5-sulphonate - 4.1 3228 ACETONITRILE - 3 1648 3-Acetoxypropene, see - 3 2333 Acetylacetone, see - 3 2310 Acetyl acetone peroxide (concentration ≤32%, as a paste), see - 5.2 3106 Acetyl acetone peroxide (concentration ≤42%, with diluent Type A, and - 5.2 3105 water, available oxygen ≤4.7%), see ACETYL BROMIDE - 8 1716 ACETYL CHLORIDE - 3 1717 Acetyl cyclohexanesulphonyl peroxide - 5.2 3115 (concentration ≤32%, with diluent Type B), see Acetyl cyclohexanesulphonyl peroxide - 5.2 3112 (concentration ≤82%, with water), see Acetylene dichloride, see - 3 1150 ACETYLENE, DISSOLVED - 2.1 1001 Acetylene, ethylene and propylene mixtures, refrigerated liquid, see - 2.1 3138 ACETYLENE, SOLVENT FREE - 2.1 3374 Acetylene tetrabromide, see P 6.1 2504 Acetylene tetrachloride, see P 6.1 1702 ACETYL IODIDE - 8 1898 Acetyl ketene, stabilized, see - 6.1 2521 ACETYL METHYL CARBINOL - 3 2621 Acid butyl phosphate, see - 8 1718 Acid mixture, hydrofluoric and sulphuric, see - 8 1786 Acid mixture, nitrating acid, see - 8 1796 Korean Register of Shipping IMDG Code (Amendment 37-14) Index Substance, material or article MP Class UN No. -

(12) Patent Application Publication (10) Pub. No.: US 2007/0020304 A1 Tamarkin Et Al
US 20070020304A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2007/0020304 A1 Tamarkin et al. (43) Pub. Date: Jan. 25, 2007 (54) NON-FLAMMABLE INSECTICDE on Nov. 29, 2002. Provisional application No. 60/696, COMPOSITION AND USES THEREOF 878, filed on Jul. 6, 2005. (75) Inventors: Dov Tamarkin, Maccabim (IL); Doron (30) Foreign Application Priority Data Friedman, Karmei Yosef (IL); Meir Eini, Ness Ziona (IL) Oct. 25, 2002 (IL)................................................. 1524.86 Correspondence Address: Publication Classification WILMER CUTLER PICKERING HALE AND DORR LLP (51) Int. Cl. 6O STATE STREET AOIN 25/00 (2006.01) BOSTON, MA 02109 (US) (52) U.S. Cl. .............................................................. 424/405 (73) Assignee: Foamix Ltd., Rehovot (IL) (57) ABSTRACT The present invention provides a safe and effective insecti (21) Appl. No.: 11/481,596 cide composition Suitable for treating a subject infested with a parasitic anthropode or to prevent infestation by an arthro (22) Filed: Jul. 6, 2006 pod. The insecticide composition is a foamable composition, Related U.S. Application Data including a first insecticide; at least one organic carrier selected from a hydrophobic organic carrier, a polar solvent, (63) Continuation-in-part of application No. 10/911,367, an emollient and mixtures thereof, at a concentration of filed on Aug. 4, 2004. about 2% to about 5%, or about 5% to about 10%; or about Continuation-in-part of application No. 10/532,618, 10% to about 20%; or about 20% to about 50% by weight; filed on Dec. 22, 2005, filed as 371 of international about 0.1% to about 5% by weight of a surface-active agent; application No. -

NMP-Free Formulations of Neonicotinoids
(19) & (11) EP 2 266 400 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 29.12.2010 Bulletin 2010/52 A01N 43/40 (2006.01) A01N 43/86 (2006.01) A01N 47/40 (2006.01) A01N 51/00 (2006.01) (2006.01) (2006.01) (21) Application number: 09305544.0 A01P 7/00 A01N 25/02 (22) Date of filing: 15.06.2009 (84) Designated Contracting States: (72) Inventors: AT BE BG CH CY CZ DE DK EE ES FI FR GB GR • Gasse, Jean-Jacques HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL 27600 Saint-Aubin-Sur-Gaillon (FR) PT RO SE SI SK TR • Duchamp, Guillaume Designated Extension States: 92230 Gennevilliers (FR) AL BA RS • Cantero, Maria 92230 Gennevilliers (FR) (71) Applicant: NUFARM 92233 Gennevelliers (FR) (74) Representative: Cabinet Plasseraud 52, rue de la Victoire 75440 Paris Cedex 09 (FR) (54) NMP-free formulations of neonicotinoids (57) The invention relates to NMP-free liquid formulation comprising at least one nicotinoid and at least one aprotic polar component selected from the group comprising the compounds of formula I, II or III below, and mixtures thereof, wherein R1 and R2 independently represent H or an alkyl group having less than 5 carbons, preferably a methyl group, and n represents an integer ranging from 0 to 5, and to their applications. EP 2 266 400 A1 Printed by Jouve, 75001 PARIS (FR) EP 2 266 400 A1 Description Technical Field of the invention 5 [0001] The invention relates to novel liquid formulations of neonicotinoids and to their use for treating plants, for protecting plants from pests and/or for controlling pests infestation. -
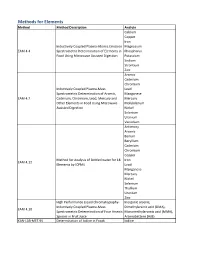
Method Description
Methods for Elements Method Method Description Analyte Calcium Copper Iron Inductively Coupled Plasma-Atomic Emission Magnesium EAM 4.4 Spectrometric Determination of Elements in Phosphorus Food Using Microwave Assisted Digestion Potassium Sodium Strontium Zinc Arsenic Cadmium Chromium Inductively Coupled Plasma-Mass Lead Spectrometric Determination of Arsenic, Manganese EAM 4.7 Cadmium, Chromium, Lead, Mercury and Mercury Other Elements in Food Using Microwave Molybdenum Assisted Digestion Nickel Selenium Uranium Vanadium Antimony Arsenic Barium Beryllium Cadmium Chromium Copper Method for Analysis of Bottled water for 18 Iron EAM 4.12 Elements by ICPMS Lead Manganese Mercury Nickel Selenium Thallium Uranium Zinc High Performance Liquid Chromatography- Inorganic arsenic, Inductively Coupled Plasma-Mass Dimethylarsinic acid (DMA), EAM 4.10 Spectrometric Determination of Four Arsenic Monomethylarsonic acid (MMA), Species in Fruit Juice Arsenobetaine (AsB) KAN-LAB-MET.95 Determination of Iodine in Foods Iodine Methods for Radionuclides Method Method Description Analyte Determination of Strontium-90 in Foods by WEAC.RN.METHOD.2.0 Strontium-90 Internal Gas-Flow Proportional Counting Americium-241 Cesium-134 Cesium-137 Determination of Gamma-Ray Emitting Cobalt-60 WEAC.RN.METHOD.3.0 Radionuclides in Foods by High-Purity Potassium-40 Germanium Spectrometry Radium-226 Ruthenium-103 Ruthenium-106 Thorium-232 Methods for Pesticides/Industrial Chemicals Method Method Description Analyte Extraction Method: Analysis of Pesticides KAN-LAB-PES.53 and -

Environmental Health Criteria 145 Methyl Parathion
Environmental Health Criteria 145 Methyl parathion Please note that the layout and pagination of this web version are not identical with the printed version. Methyl parathion (EHC 145, 1992) INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY ENVIRONMENTAL HEALTH CRITERIA 145 METHYL PARATHION This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the International Labour Organisation, or the World Health Organization. Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organisation, and the World Health Organization First draft prepared by Dr R.F. Hertel and co-workers, Fraunhofer Institute of Toxicology and Aerosol Research, Hanover, Germany World Health Orgnization Geneva, 1993 The International Programme on Chemical Safety (IPCS) is a joint venture of the United Nations Environment Programme, the International Labour Organisation, and the World Health Organization. The main objective of the IPCS is to carry out and disseminate evaluations of the effects of chemicals on human health and the quality of the environment. Supporting activities include the development of epidemiological, experimental laboratory, and risk-assessment methods that could produce internationally comparable results, and the development of manpower in the field of toxicology. Other activities carried out by the IPCS include the development of know-how for coping with chemical accidents, -

United States Patent (19) 11 Patent Number: 5,232,484 Pignatello 45 Date of Patent: Aug
USOO5232484A United States Patent (19) 11 Patent Number: 5,232,484 Pignatello 45 Date of Patent: Aug. 3, 1993 (54). DEGRADATION OF PESTICIDES BY Waste Disposal Proceedings EPA/600/9-85/030, pp. FERRC REAGENTS AND PEROXDEN 72 to 85. THE PRESENCE OF LIGHT Johnson, L. J., and Talbot, H. W., 39 Experimentia 1236-1246 (1983). 75 Inventor: Joseph J. Pignatello, Hamden, Conn. Mill, T., and Haag, W. R., Preprints of Extended Ab 73 Assignee: The Connecticut Agricultural stracts, 198th Nat. Meeting Amer. Chem. Soc. Div. Experiment Station, New Haven, Env, Chem., A.C.S., Washington, 1969, paper 155, pp. Conn. 342-345. 21 Appl. No.: 744,365 Munnecke, D. M., 70 Residue Rev. 1-26 (1979). Murphy, A. P., et al., 23 Environ. Sci. Technol. 166-169 22 Filed: Aug. 13, 1991 (1989). 51 int. Ci...................... A01N 37/38; A01N 43/70; Nye, J. C., 1985 National Workshop on Pesticide Waste CO2F 1/72 Disposal Proceedings EPA/600/9-85/030 pp. 43 to 52 U.S.C. .................................... 588/206; 210/759; 480. 588/207; 588/210 Plimmer, J. R., et al., 19 J.Agr. Food Chem. 572-573 58 Field of Search ........................... 71/117, 116,93; (1971). 210/749, 758, 759 Schmidt, C., 1986 National Workshop on Pesticide Waste Disposal Proceedings EPA/600/9-87/001, pp. 56) References Cited 45 to 52. U.S. PATENT DOCUMENTS Sedlak, D. L. and Andren, A. W., 25 Environ. Sci. 3,819,516 6/1974 Murchison et al. ................... 422A24 Technol. 777-782 (1991). 4,569,769 2/1986 Walton et al. -

Acetylcholinesterase
AChE Acetylcholinesterase Acetylcholinesterase (AChE or acetylhydrolase) is a hydrolase that hydrolyzes the neurotransmitter acetylcholine. AChE is found at mainly neuromuscular junctions and cholinergic brain synapses, where its activity serves to terminate synaptic transmission. It belongs tocarboxylesterase family of enzymes. It is the primary target of inhibition by organophosphorus compounds such as nerve agents and pesticides. AChE has a very high catalytic activity - each molecule of AChE degrades about 25000 molecules ofacetylcholine (ACh) per second, approaching the limit allowed by diffusion of the substrate. ACh is released from the nerve into the synaptic cleft and binds to ACh receptors on the post-synaptic membrane, relaying the signal from the nerve. AChE, also located on the post-synaptic membrane, terminates the signal transmission by hydrolyzing ACh. The liberated choline is taken up again by the pre-synaptic nerve and ACh is synthetized by combining with acetyl-CoA through the action of choline acetyltransferase. www.MedChemExpress.com 1 AChE Inhibitors & Activators (+)-Phenserine (-)-Corynoxidine Cat. No.: HY-16009 Cat. No.: HY-N7010 (+)-Phenserine is a novel selective cholinesterase (-)-Corynoxidine is an acetylcholinesterase noncompetitive inhibitor with an IC50 of 45.3 μM. inhibitor with an IC50 value of 89.0 μM, isolated from the aerial parts of Corydalis speciosa. (-)-Corynoxidine exhibits antibacterial activities against Staphylococcus aureus and methicillin-resistant S. Purity: 98.09% Purity: >98% Clinical Data: No Development Reported Clinical Data: No Development Reported Size: 5 mg, 10 mg, 50 mg Size: 1 mg, 5 mg (-)-Huperzine A (R)-Rivastigmine D6 tartrate (Huperzine A) Cat. No.: HY-17387 Cat. No.: HY-11017AS (-)-Huperzine A (Huperzine A) is an alkaloid (R)-Rivastigmine D6 tartrate is the deuterium isolated from a Chinese club moss, with labeled (R)-Rivastigmine, which is an neuroprotective activity.