242 Part 522—Implantation Or Injectable Dosage Form
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pharmaceutical Starting Materials/Essential Drugs
BULLETIN MNS October 2009 PHARMACEUTICAL STARTING MATERIALS MARKET NEWS SERVICE (MNS) BI -MONTHLY EDITION Market News Service Pharmaceutical Starting Materials/Essential Drugs October 2009, Issue 5 The Market News Service (MNS) is made available free of charge to all Trade Support Institutions and enterprises in Sub-Saharan African countries under a joint programme of the International Trade Centre and CBI, the Dutch Centre for the Promotion of Imports from Developing Countries (www.cbi.nl). Should you be interested in becoming an information provider and contributing to MNS' efforts to improve market transparency and facilitate trade, please contact us at [email protected]. This issue continues the series, started at the beginning of the year, focusing on the leading markets in various world regions. This issue covers the trends and recent developments in eastern European pharmaceutical markets. To subscribe to the report or to access MNS reports directly online, please contact [email protected] or visit our website at: http://www.intracen.org/mns. Copyright © MNS/ITC 2007. All rights reserved 1 Market News Service Pharmaceutical Starting Materials Introduction WHAT IS THE MNS FOR PHARMACEUTICAL STARTING MATERIALS/ESSENTIAL DRUGS? In 1986, the World Health Assembly laid before the Organization the responsibility to provide price information on pharmaceutical starting materials. WHA 39.27 endorsed WHO‟s revised drug strategy, which states “... strengthen market intelligence; support drug procurement by developing countries...” The responsibility was reaffirmed at the 49th WHA in 1996. Resolution WHA 49.14 requests the Director General, under paragraph 2(6) “to strengthen market intelligence, review in collaboration with interested parties‟ information on prices and sources of information on prices of essential drugs and starting material of good quality, which meet requirements of internationally recognized pharmacopoeias or equivalent regulatory standards, and provide this information to member states”. -

Pharmacology on Your Palms CLASSIFICATION of the DRUGS
Pharmacology on your palms CLASSIFICATION OF THE DRUGS DRUGS FROM DRUGS AFFECTING THE ORGANS CHEMOTHERAPEUTIC DIFFERENT DRUGS AFFECTING THE NERVOUS SYSTEM AND TISSUES DRUGS PHARMACOLOGICAL GROUPS Drugs affecting peripheral Antitumor drugs Drugs affecting the cardiovascular Antimicrobial, antiviral, Drugs affecting the nervous system Antiallergic drugs system antiparasitic drugs central nervous system Drugs affecting the sensory Antidotes nerve endings Cardiac glycosides Antibiotics CNS DEPRESSANTS (AFFECTING THE Antihypertensive drugs Sulfonamides Analgesics (opioid, AFFERENT INNERVATION) Antianginal drugs Antituberculous drugs analgesics-antipyretics, Antiarrhythmic drugs Antihelminthic drugs NSAIDs) Local anaesthetics Antihyperlipidemic drugs Antifungal drugs Sedative and hypnotic Coating drugs Spasmolytics Antiviral drugs drugs Adsorbents Drugs affecting the excretory system Antimalarial drugs Tranquilizers Astringents Diuretics Antisyphilitic drugs Neuroleptics Expectorants Drugs affecting the hemopoietic system Antiseptics Anticonvulsants Irritant drugs Drugs affecting blood coagulation Disinfectants Antiparkinsonian drugs Drugs affecting peripheral Drugs affecting erythro- and leukopoiesis General anaesthetics neurotransmitter processes Drugs affecting the digestive system CNS STIMULANTS (AFFECTING THE Anorectic drugs Psychomotor stimulants EFFERENT PART OF THE Bitter stuffs. Drugs for replacement therapy Analeptics NERVOUS SYSTEM) Antiacid drugs Antidepressants Direct-acting-cholinomimetics Antiulcer drugs Nootropics (Cognitive -

Execution by . . . Heroin?: Why States Should Challenge the FDA's Ban
N2_BERGS_UPDATED (DO NOT DELETE) 1/15/2017 9:55 AM Execution by . Heroin?: Why States Should Challenge the FDA’s Ban on the Importation of Sodium Thiopental Matthew C. Bergs* ABSTRACT: This Note traces the history of the lethal injection drug shortage and its impact on how states carry out the death penalty. Though this topic has received much attention in recent years, relatively little attention has been paid to the D.C. Circuit’s decision in Cook v. FDA, which exacerbated the shortage. In Cook, the D.C. Circuit enjoined the FDA from exercising its enforcement discretion to allow the importation of sodium thiopental, the primary anesthetic used by states in lethal injection executions. This decision is significant because it effectively required the FDA to ban the importation of sodium thiopental, forcing states to find other ways to carry out executions. Many states have turned to new drugs and manufacturers, while others have returned to past methods of execution. However, states’ use of alternative drugs and manufacturers has had disastrous consequences, and the return to old methods of execution constitutes an unacceptable regression towards inhumane and barbaric punishment. Thus, this Note argues that states should make every effort to obtain sodium thiopental. Potential avenues to obtain the drug include adhering to the FDA’s regulations or litigating against the FDA’s regulations. Renewed access to sodium thiopental will resolve many of the problems plaguing the administration of lethal injection. I. INTRODUCTION & BACKGROUND .................................................. 762 A. HISTORY OF LETHAL INJECTION AS A METHOD OF EXECUTION ... 763 B. DEVELOPMENT OF THE LETHAL INJECTION DRUG SHORTAGE .... -

IV Induction Agents
Intravenous drugs used for the induction of anaesthesia Dr Tom Lupton, Specialist Registrar in Anaesthesia Dr Oliver Pratt, Consultant Anaesthetist Salford Royal Hospitals NHS Foundation Trust, Salford, UK Key questions This tutorial reviews the basic pharmacology of common intravenous (IV) anaesthetic drugs. By the end of the tutorial, you should be able to decide on the most appropriate drug to use in the situations below and for what reason: 1. A patient with intestinal obstruction requires an emergency laparotomy. 2. A patient with a history of throat cancer, showing marked stridor and signs of respiratory distress, requires a tracheostomy. 3. A patient requiring a burn dressing change. 4. A patient with a history of heart failure requires a general anaesthetic. 5. A dehydrated hypovolaemic patient requires an emergency general anaesthetic. 6. A patient with porphyria comes for an inguinal hernia repair and is requesting a general anaesthetic. 7. A patient requires sedation on the intensive care unit. 8. Anaesthesia in the prehospital environment. What are IV induction drugs? These are drugs that, when given intravenously in an appropriate dose, cause a rapid loss of consciousness. This is often described as occurring within “one arm-brain circulation time” that is simply the time taken for the drug to travel from the site of injection (usually the arm) to the brain, where they have their effect. They are used: • To induce anaesthesia prior to other drugs being given to maintain anaesthesia. • As the sole drug for short procedures. • To maintain anaesthesia for longer procedures by intravenous infusion. • To provide sedation. The concept of intravenous anaesthesia was born in 1932, when Wesse and Schrapff published their report into the use of hexobarbitone, the first rapidly acting intravenous drug. -

G/LIC/N/3/VEN/1 27 May 2002 ORGANIZATION (02-2903)
WORLD TRADE G/LIC/N/3/VEN/1 27 May 2002 ORGANIZATION (02-2903) Committee on Import Licensing Original: Spanish AGREEMENT ON IMPORT LICENSING PROCEDURES1 Notification under Article 7.3 of the Agreement VENEZUELA The following communication, dated 16 May 2002, has been received from the Permanent Mission of the Bolivarian Republic of Venezuela. _______________ I. ADMINISTRATION OF TARIFF QUOTAS FOR A SERIES OF AGRICULTURAL ITEMS......................................................................................................1 II. IMPORT LICENCES FOR ENVIRONMENTAL PROTECTION PURPOSES ..............5 III. IMPORT LICENCES FOR PUBLIC HEALTH PURPOSES .............................................7 IV. IMPORT LICENCES FOR STATE SECURITY PURPOSES..........................................10 V. IMPORT LICENCES FOR SLOT MACHINES, ACCESSORIES AND EQUIPMENT..........................................................................................................................12 VI. IMPORT LICENCES FOR NEW SCRAPS FROM THE CLOTHING INDUSTRY..............................................................................................................................14 ANNEX 12 .............................................................................................................................................16 I. ADMINISTRATION OF TARIFF QUOTAS FOR A SERIES OF AGRICULTURAL ITEMS Outline of systems 1. System of administration of tariff quotas for a series of agricultural items, in accordance with the minimum access commitments made by Venezuela -

218 Part 522—Implantation Or Injectable Dosage Form
Pt. 522 21 CFR Ch. I (4–1–07 Edition) by Mycoplasma gallisepticum sensitive 522.82 Aminopropazine fumarate sterile so- to tylosin. lution injection. (iii) Limitations. Do not use in layers 522.84 Beta-aminopropionitrile fumarate. 522.88 Sterile amoxicillin trihydrate for sus- producing eggs for human consump- pension. tion; administer from 2 to 5 days as 522.90 Ampicillin implantation and sole source of drinking water; treated injectible dosage forms. turkeys should consume enough medi- 522.90a Ampicillin trihydrate sterile suspen- cated drinking water to provide 60 mil- sion. ligrams of tylosin per pound of body 522.90b Ampicillin trihydrate for sterile sus- weight per day; prepare a fresh solu- pension. tion every 3 days; when sinus swelling 522.90c Ampicillin sodium for aqueous injec- tion. is present, inject the sinus with tylosin 522.144 Arsenamide sodium aqueous injec- injectable simultaneously with the tion. drinking water treatment; do not ad- 522.147 Atipamezole. minister within 5 days of slaughter. 522.150 Azaperone injection. (3) Swine—(i) Amount. 0.25 gram per 522.161 Betamethasone acetate and gallon. betamethasone disodium phosphate aque- (ii) Indications for use. For the control ous suspension. 522.163 Betamethasone dipropionate and and treatment of swine dysentery betamethasone sodium phosphate aque- (bloody scours) caused by pathogens ous suspension. sensitive to tylosin. 522.204 Boldenone. (iii) Limitations. As only source of 522.234 Butamisole hydrochloride. drinking water for 3 to 10 days, depend- 522.246 Butorphanol tartrate injection. ing on the severity of the condition 522.275 N-Butylscopolammonium bromide. being treated: mix fresh solution daily; 522.311 Carfentanil citrate injection. -

Pharmacology Cito
Pharmacology Cito 5 Read: it is important! Dear future physicians and pharmaceutists! The scientific and pedagogical staff of the Pharmacology department of the National University of Pharmacy (Kharkiv) presents a new textbook "Pharmacology – Cito!" to you. We want to change the myth that it is impossible to learn pharmacology quickly and qualitatively. The textbook "Pharmacology – Cito!" is published for those, who have decided to connect his or her profession with medicines, but think that a great amount of the information in pharmacology is hard to learn. Taking this fact into account we have decided to help those, who wish to master pharmacology in logical, fast - "Cito!"- version. Not be afraid of pharmacology! The 40-year experience of teaching pharmacology testifies that for the last 10-15 years a tendency of reducing the amount of classroom hours in pharmacology because of increasing the amount of the individual work is being observed. However, because of the lack of time and the constant increasing volume of information in pharmacology, everyone has not enough time to master it soundly and qualitatively. This textbook trains future pharmaceutists and physicians in the pharmacological logic, i.e. knowing the mechanisms of drug action one can understand their pharmacodynamics, naturally, on the basis of pharmacodynamics one can find logically the indications to their application and from their side effects the contraindications can be seen. The information about the peculiarities of medicines has been generalized and given as a pharmacological ―face‖ in tables. The volume of this textbook in pharmacology is sufficient for acquiring the confidence in the opportunity of the further improving of knowledge in this discipline, which is important for a physician and a pharmaceutist. -
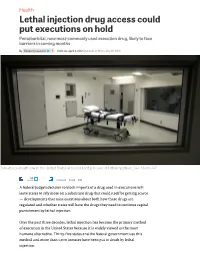
Lethal Injection Drug Access Could Put Executions on Hold Pentobarbital, Now Most-Commonly Used Execution Drug, Likely to Face Barriers in Coming Months
Health Lethal injection drug access could put executions on hold Pentobarbital, now most-commonly used execution drug, likely to face barriers in coming months By Kimberly Leonard 6:00 am, April 4, 2012 Updated: 12:19 pm, May 19, 2014 Inmates on death row in the United States are executed by means of lethal injection. Dave Martin/AP 55 tweets Comment E-mail Print A federal judge’s decision to block imports of a drug used in executions will leave states to rely more on a substitute drug that could itself be getting scarce — developments that raise questions about both how these drugs are regulated and whether states will have the drugs they need to continue capital punishment by lethal injection. Over the past three decades, lethal injection has become the primary method of execution in the United States because it is widely viewed as the most humane alternative. Thirty-five states and the federal government use this method and more than 1,100 inmates have been put to death by lethal injection. State justice or corrections departments have conducted these executions by administering the anesthetic sodium thiopental in a lethal dosage on its own, or as part of a three-step “cocktail” in which sodium thiopental is followed by pancuronium bromide, a paralytic agent, then potassium chloride, which stops the heart and causes death. But in late March, a federal judge blocked importation of sodium thiopental, ruling that the Food and Drug Administration (FDA) ignored the law by allowing it to be imported into the country without following regulatory protocol. -

Differential Effects of Halothane and Thiopental on Surfactant Protein C
805 Anesthesiology 2000; 93:805–10 © 2000 American Society of Anesthesiologists, Inc. Lippincott Williams & Wilkins, Inc. Differential Effects of Halothane and Thiopental on Surfactant Protein C Messenger RNA In Vivo and In Vitro in Rats Catherine Paugam-Burtz, M.D.,* Serge Molliex, M.D, Ph.D.,† Bernard Lardeux, Ph.D.,‡ Corinne Rolland, T.A.,§, Michel Aubier, M.D,ʈ Jean-Marie Desmonts, M.D.,# Bruno Crestani, M.D., Ph.D.** Downloaded from http://pubs.asahq.org/anesthesiology/article-pdf/93/3/805/401255/0000542-200009000-00030.pdf by guest on 28 September 2021 Background: Pulmonary surfactant is a complex mixture of lung SP-C mRNA content to 53% of the value obtained in control proteins and phospholipids synthetized by alveolar type II (nonanesthetized, nonventilated) animals; thiopental anesthe- cells. Volatile anesthetics have been shown to reduce surfactant sia increased to 150% the lung SP-C mRNA content. phospholipid biosynthesis by rat alveolar type II cells. Surfac- Conclusions: These findings indicate that halothane and thio- tant-associated protein C (SP-C) is critical for the alveolar sur- pental used at clinically relevant concentrations modulate the factant functions. Our goal was to evaluate the effects of halo- pulmonary SP-C mRNA content in rats. In vivo, the additive role thane and thiopental on SP-C messenger RNA (mRNA) of mechanical ventilation is suggested. (Key words: Lung; pneu- expression in vitro in rat alveolar type II cells and in vivo in mocytes, volatile anesthetics.) mechanically ventilated rats. Methods: In vitro, freshly isolated alveolar type II cells were exposed to halothane during 4 h (1, 2, 4%) and 8 h (1%), and to PULMONARY surfactant is a complex mixture of pro- thiopental during 4 h (10, 100 M) and 8 h (100 M). -

Administrative Records, Texas Department of Criminal Justice
Consistent with the terms of the Court’s May 22, 2017 scheduling order, the record has been redacted for all information that plaintiff, Texas Department of Criminal Justice (Texas), has identified as confidential. In addition, Defendants have also redacted information that the drug’s supplier and broker have separately advised the agency they consider confidential and private, as well as information the agency itself generally treats as confidential. This information has been redacted pending final FDA’s review of confidentiality claims, and our filing of the record with these redactions does not necessarily reflect our agreement with all of the claims of confidentiality Defendants have received. Defendants explicitly reserve the right to make an independent determination regarding the proper scope of redactions at a later time. Should we identify any of Texas’s redactions that are over-broad or otherwise improper, we will work with Texas’s counsel to revise the redactions in the record. MISCELLANEOUS FDA 568 DOCUMENT#: VERSION#: DIOP PROCEDURE ~L ORO-DIOP- 2.00 FOOD & DRUG ADMINISTRATION .008 I OFFICE OF REGULATORY AFFAIRS PAGE 1 OF 4 TITLE: EFFECTIVE DATE: PROCESSING OF SODIUM THIOPENTAL ENTRIES 4/16/2012 Sections included in this document 1. Purpose 2. Scope 3. Responsibility 4. Background 5. References 6. Procedure 7. Definitions/Glossary 8. Records 9. Supporting Documents 10. Contact Information 11.Attachments Document History and Change History 1. Purpose Pending litigation, this procedure provides instructions on the handling of shipments of Sodium Thiopental by Districts and the responsibility of DIOP in the processing of Sodium Thiopental entries. Districts should disseminate this information to all pertinent staff handling these entries. -

ANESTHESIA and ANALGESIA in LABORATORY ANIMALS, 2ND EDITION Copyright © 2008, 1997 by Elsevier Inc
Anesthesia and Analgesia in Laboratory Animals AMERICAN COLLEGE OF LABORATORY ANIMAL MEDICINE SERIES Steven H. Weisbroth, Ronald E. Flatt, and Alan L. Kraus, eds.: The Biology of the Laboratory Rabbit, 1974 Joseph E. Wagner, and Patrick J. Manning, eds.: The Biology of the Guinea Pig, 1976 Edwin J. Andrews, Billy C. Ward, and Norman H. Altman, eds.: Spontaneous Animal Models of Human Disease, volume 1, 1979 Edwin J. Andrews, Billy C. Ward, and Norman H. Altman, eds.: Spontaneous Animal Models of Human Disease, volume 2, 1980 Henry J. Baker, J. Russell Lindsey, and Steven H. Weisbroth, eds.: The Laboratory Rat, Volume I: Biology and Diseases, 1979; Volume II: Research Applications, 1980 Henry L. Foster, J. David Small, and James G. Fox, eds.: The Mouse in Biomedical Research, Volume I: History, Genetics and Wild Mice, 1981, Volume II: Diseases, 1982; Vol. III: Normative Biology, Immunology and Husbandry, 1983; Vol. IV: Experimental Biology and Oncology, 1982 James G. Fox, Bennett J. Cohen, and Franklin M. Loew, eds.: Laboratory Animal Medicine, 1984 G.L. Van Hoosier, Jr., and Charles W. McPherson, eds.: Laboratory Hamsters, 1987 Patrick J. Manning, Daniel H. Ringler, and Christian E. Newcomer, eds.: The Biology of the Laboratory Rabbit, second edition, 1994 B. Taylor Bennett, Christian R. Abee, Roy Henrickson, eds: Nonhuman Primates in Biomedical Research, Biology and Management, 1995 Dennis F. Kohn, Sally K. Wixson, William J. White, and G. John Benson, eds.: Anesthesia and Analgesia in Laboratory Animals, 1997 B. Taylor Bennett, Christian R. Abee, Roy Henrickson, eds: Nonhuman Primates in Biomedical Research, Diseases, 1998 James G. Fox, Lynn C. -

Anesthesia • Cattle
Anesthesia 1n• Cattle N~ Kent Ames, D. V.M., M .S. Thomas W. Reibold, D. V.M. Dept. of Large Animal Surgery and Medicine Michigan State University East Lansing, Michigan 48824 Due to the nature of cattle, most surgical Regional nerve blocks in calves may be more dif procedures can be accomplishe1 with physical ficult due to slightly different anatomy and confor restraint and local anesthesia. This is an excellent mation in the growing animal. Procaine toxicity method for most surgical procedures, but what about can also be a problem if the amount of procaine ex the procedures where more complete anesthesia is ceeds 2.7 mg/kg (6.0 mg/lb.) in calves (5). desired or required? How often is a surgical procedure not performed or performed inadequately because it General Anesthesia would require the use of general anesthesia? The concepts of general anesthesia in cattle are very similar to anesthesia in other animals, except for Local and, Regional Anesthesia a few species variations. Prior to anesthetizing any First, let us look at the use of local and regional animal, a few precautions are necessary. A routine anesthesia. Local anesthetic agents prevent the con physical exam should be performed to determine the duction of nerve impulses along the nerves and thus, state of health of the animal. Particular attention the patient perceives no pain. The agents themselves should be paid to the cardiopulmonary system. Basic are weak acids, and do not work well in an acid clinical pathology tests should be performed to obtain medium such as an abscess.