Long-Term Monitoring at the East and West Flower Garden Banks
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

St. Kitts Final Report
ReefFix: An Integrated Coastal Zone Management (ICZM) Ecosystem Services Valuation and Capacity Building Project for the Caribbean ST. KITTS AND NEVIS FIRST DRAFT REPORT JUNE 2013 PREPARED BY PATRICK I. WILLIAMS CONSULTANT CLEVERLY HILL SANDY POINT ST. KITTS PHONE: 1 (869) 765-3988 E-MAIL: [email protected] 1 2 TABLE OF CONTENTS Page No. Table of Contents 3 List of Figures 6 List of Tables 6 Glossary of Terms 7 Acronyms 10 Executive Summary 12 Part 1: Situational analysis 15 1.1 Introduction 15 1.2 Physical attributes 16 1.2.1 Location 16 1.2.2 Area 16 1.2.3 Physical landscape 16 1.2.4 Coastal zone management 17 1.2.5 Vulnerability of coastal transportation system 19 1.2.6 Climate 19 1.3 Socio-economic context 20 1.3.1 Population 20 1.3.2 General economy 20 1.3.3 Poverty 22 1.4 Policy frameworks of relevance to marine resource protection and management in St. Kitts and Nevis 23 1.4.1 National Environmental Action Plan (NEAP) 23 1.4.2 National Physical Development Plan (2006) 23 1.4.3 National Environmental Management Strategy (NEMS) 23 1.4.4 National Biodiversity Strategy and Action Plan (NABSAP) 26 1.4.5 Medium Term Economic Strategy Paper (MTESP) 26 1.5 Legislative instruments of relevance to marine protection and management in St. Kitts and Nevis 27 1.5.1 Development Control and Planning Act (DCPA), 2000 27 1.5.2 National Conservation and Environmental Protection Act (NCEPA), 1987 27 1.5.3 Public Health Act (1969) 28 1.5.4 Solid Waste Management Corporation Act (1996) 29 1.5.5 Water Courses and Water Works Ordinance (Cap. -

Cruise Report W-48 Scientific Activities Undertaken Aboard R/V Westward Woods Hole
Cruise Report W-48 Scientific Activities Undertaken Aboard R/V Westward Woods Hole - St. Thomas 10 October - 21 November 1979 ff/lh Westward (R.Long) • Sea Education Association - Woods Hole, Massachusetts " CRUISE REPORT W-48 Scientific Activities Woods Hole - Antigua - St. Lucia - Bequia - St. Thomas 10 October 1979 - 21 November 1979 R/V Westward Sea Education Association ',,, Woods Hole, Massachusetts .. SHIPBOARD DRAFT .. ----------------------- - ( PREFACE This Cruise Report is written in an attempt to accomplish two objectives. Firstly, and more importantly, it presents a brief outline of the scientific research completed aboard R/V Westward during W-48. Reports of the status of on-going projects and of the traditional academic program are presented. In addition, abstracts from the research projects of each student are included. Secondly, for those of us that participated, it represents the product of our efforts and contains a record of other events that were an important part of the trip, in particular the activities during port stops. Once again, lowe special thanks to Abby Ames, who was in charge of the shipboard laboratory, and upon whom I was able to depend through out the cruise. Her effectiveness and perseverance under the difficult working conditions at sea, and her cheerful attitude and enthusiasm were greatly appreciated by us all. Rob Nawojchik, who participated as an Assistant Scientist, added a new field of interest to the cruise with his vast knowledge of ichthyology. The energy with which he pursued his interest and his enthusiasm for the subject, set an example for us all. Two visiting scholars participated in different legs of this cruise. -

Bleaching and Related Ecological Factors CRTR Working Group Findings 2004-2009 Bleaching and Related Ecological Factors: CRTR Working Group Findings 2004-2009
Bleaching and Related Ecological Factors CRTR Working Group Findings 2004-2009 Bleaching and Related Ecological Factors: CRTR Working Group Findings 2004-2009 Acknowledgements The Bleaching Working Group is grateful for funding provided by the World Bank and the Global Environment Facility. The members are also grateful for the effi cient administration of the project provided by Ms Melanie King, Ms Lianne Cook, and members of the CRTR Project Executing Agency (PEA) at the University of Queensland. They thank Ms Catalina Reyes-Nivia for coordinating the production of this report. Contributing Authors: Ove Hoegh-Guldberg1, Yossi Loya2, John Bythell3, William Fitt4, Ruth Gates5, Roberto Iglesias-Prieto6, Michael Lesser7, Tim McClanahan8, Robert van Woesik9, Christian Wild10 Cover Photo: Ove Hoegh-Guldberg 1University of Queensland, 2Tel Aviv University, 3University of Newcastle, 4University of Georgia, 5University of Hawaii, 6Universidad Nacional Autónoma of México, 7University of New Hampshire, 8Wildlife Conservation Society, 9Florida Institute of Technology, 10University of Munich. The Coral Reef Targeted Research & Capacity Building for Management (CRTR) Program is a leading international coral reef research initiative that provides a coordinated approach to credible, factual and scientifi cally-proven knowledge for coral reef management. The CRTR Program is a partnership between the Global Environment Facility, the World Bank, The University of Queensland (Australia), the United States National Oceanic and Atmospheric Administration (NOAA) and approximately 50 research institutes and other third-parties around the world. Contact: Coral Reef Targeted Research & Capacity Building for Management Program, c/- Centre for Marine Studies, Gerhmann Building, The University of Queensland, St Lucia, Qld 4072, Australia Tel: +61 7 3346 9942 Fax: +61 7 3346 9987 Email: [email protected] Internet: www.gefcoral.org Product code: 004/2009 Editorial design and production: Currie Communications, Melbourne, Australia, October 2009. -

Reef Fish Amendment 14
09/23/96 AMENDMENT 14 TO THE FISHERY MANAGEMENT PLAN FOR THE REEF FISH FISHERY OF THE GULF OF MEXICO (Includes Regulatory Impact Review, Initial Regulatory Flexibility Analysis, and Environmental Assessment) AUGUST 1996 GULF OF MEXICO FISHERY MANAGEMENT COUNCIL LINCOLN CENTER, SUITE 331 5401 WEST KENNEDY BOULEVARD TAMPA, FLORIDA 33609 (813) 228-2815 FAX (813) 225-7015 This is a publication ofthe GulfofMexico Fishery Management Council pursuant to National Oceanic and Atmospheric Award No. NA67FC0002. Abbreviations Used in This Document AP Advisory Panel SMZ Special Management Zone Council GulfofMexico Fishery SPR Spawning Potential Ratio Management Council SSBR Spawning Stock Biomass Per EEZ Exclusive Economic Zone Recruit B.O. Executive Order SSC Scientific and Statistical Committee FMFC Florida Marine Fisheries TAC Total Allowable Catch Commission FMP Fishery Management Plan GMFMC GulfofMexico Fishery Management Council IRFA Initial Regulatory Flexibility Analysis ITQ Individual Transferable Quota NMFS National Marine Fisheries Service NOAA National Oceanic and Atmospheric Administration RD Regional Director ofNMFS RFA Regulatory Flexibility Act of 1980 RIR Regulatory Impact Review SAFMC South Atlantic Fishery Management Council SBA Small Business Administration SEFSC Southeast Fisheries Science Center ofNMFS SEIS Supplemental Environmental Impact Statement SEP Socioeconomic Panel i CONTENTS REEF FISH AMENDMENT 14 1.0 INTRODUCTION. ......................................................... .. 1 2.0 mSTORY OF MANAGEMENT ............................................... .. 1 3.0 PURPOSE AND NEED FOR ACTION. ......................................... .. 5 4.0 PROBLEMS REQUIRING A PLAN AMENDMENT 5 5.0 PROPOSED ACTIONS 6 6.0 FISH TRAP ENDORSEMENT MORATORIUM .................................. .. 7 6.1 Introduction 7 6.2 History ofFish Trap Regulation 7 6.3 Summary ofResearch on GulfFish Trap Fishery 8 OBSERVER STUDY 9 Methodology and Sampling Protocol .............................. -

Kirra Reef Biota Monitoring Report 2016
Tweed River Entrance Sand Bypassing Project Kirra Reef Biota Monitoring 2016 Final Report New South Wales Department of Industry ecology / vegetation / wildlife / aquatic ecology / GIS Executive summary New South Wales Department of Industry has commissioned Ecosure Pty Ltd to undertake the 2016 Kirra Reef biota monitoring program, where the project will provide assessment to adequately identify and describe the residing flora and fauna communities of Kirra Reef and three control sites to both compare and build on the existing monitoring program. Benthic assemblages Differences in the composition (percent coverage and type of taxon) of benthic assemblages, algal assemblages and faunal assemblages were each compared between horizontal and vertical surfaces among Kirra, Palm Beach, Cook Island and Kingscliff Reefs. Generally, the composition of the entire benthic assemblages differed at a range of spatial scales, with clear differences evident between surface orientations and among most reefs, except for between vertical surfaces on Kingscliff and Cook Island. The differences were primarily due to differences in the higher coverage of turf algae on horizontal surfaces, which dominated the assemblages. Similar patterns were found for faunal assemblages alone, with assemblages generally having greater diversity on vertical than horizontal surfaces. The least diverse assemblages of benthic fauna were found on Kirra Reef. Changes made to the monitoring program, particularly the increased number of control locations and identification of benthic taxa to a finer taxonomic scale, have allowed for improved understanding knowledge of the natural variation in coverage of benthic assemblages across a broader spatial scale. Taking this into account, the assemblages on Kirra Reef remain dissimilar to the comparative reefs than would be expected naturally (i.e. -

At Bimini, Bahamas
SPATIAL AND TEMPORAL VARIATION IN DIET AND PREY PREFERENCE OF NURSERY-BOUND JUVENILE LEMON SHARKS (Negapri011 brevirostris) AT BIMINI, BAHAMAS. By STEVEN PAUL NEWMAN A thesis submitted to the University of Plymouth In partial fulfilment for the degree of DOCTOR OF PIDLOSPHY Department of Biological Sciences Faculty of Science In collaboration with Bimini Biological Field Station, Bahamas October 2003 Ill ,. I l!JNIVEF.tSITY ()F ,PLVM0llJliH: UeniNo.. ~j00·58} ~84-4 I pate I 0~5; MAY 2004 I • !Class No. -T+IESIS 9\Rik-~li ,.u; . ' w. I 'coR I. No. I' I Pll'ZMOUi'H LIB!=IAAV: I I Abstract Spatial and temporal variation in the diet and prey preference of juvenile lemon sharks Prey selection has never been determined in an elasmobranch, primarily because of the large home ranges possessed by adults making accurate quantification of prey in the environment problematic. Juvenile lemon sharks spend their first few years of life within protected nursery grounds, enabling the first quantification of prey selection due to the restricted area that they inhabit. Growth and survival of juvenile lemon sharks strongly influences adult fitness and recruitment, and therefore prey selection may play an important role in the life history of lemon sharks. The selection of a preferred species or size of prey by juvenile lemon sharks was determined by comparing the proportions of prey in the diet with proportions of prey in the environment at Bimini, Bahamas, between March 2000 and March 2003. The diet of lemon sharks was quantitatively described from the analysis of 642 shark stomachs (54.7 ± 0.3 cm precaudal length PCL, mean ± S.E., range 43.5 to 90.0 cm), of which 396 (62 %) contained food items. -

Reef Manager's Guide to Coral Bleaching
A Reef Manager’s Guide to CORAL BLEACHING Paul Marshall and Heidi Schuttenberg The designation of geographical entities in this book, and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of GBRMPA, NOAA, IUCN or other participating organisations concerning the legal status of any country, territory or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. The views expressed in this publication do not necessarily reflect those of IUCN, US EPA or other participating organisations. This publication has been made possible in part by funding from the US National Oceanic and Atmospheric Administration and IUCN - The World Conservation Union. Published by: Great Barrier Reef Marine Park Authority,Townsville, Australia. Copyright: © 2006 Great Barrier Reef Marine Park Authority Reproduction of this publication for educational or other non-commercial purposes is authorised without prior written permission from the copyright holder provided the source is fully acknowledged. Reproduction of this publication for resale or other commercial purposes is prohibited without prior written permission of the copyright holder. Unless otherwise noted, photographs are copyright GBRMPA. National Library of Australia Cataloguing-in-Publication data: Marshall, Paul, 1969 A reef manager’s guide to coral bleaching. Bibliography. ISBN 1 876945 40 0 I. Coral reef conservation. 2. Coral reef ecology. 3. Coral reefs and islands. I. Schuttenberg, Heidi, 1973-II.Title. 639.972 Cover design and layout by: AECgroup Available from: Great Barrier Reef Marine Park Authority 2-68 Flinders St, PO Box 1379,Townsville, Qld 4810, Australia Tel: +61 7 4750 0700, Fax: +61 7 4772 6093 Email: [email protected] IUCN Publications Services Unit 219c Huntingdon Road, Cambridge CB3 0DL, United Kingdom Tel: +44 1223 277894, Fax: +44 1223 277175 E-mail: [email protected] www.iucn.org/bookstore A catalogue of IUCN publications is also available. -
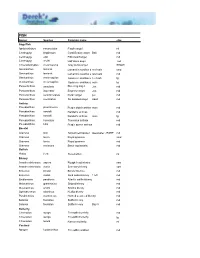
Genus Species Common Name Size Angelfish Apolemichthys
FISH Genus Species Common name size Angelfish Apolemichthys trimaculatus Flagfin angel ml Centropyge bispinosus Coral Beauty angel Bali md Centropyge eibli Eibli dwarf angel md Centropyge vroliki Half black angel md Chaetodontoplus melanosoma Gray poma angel SHOW Genicanthus lamarck Lamarck's swallow angelreef safe smd Genicanthus lamarck Lamarck's swallow angelreef safe md Genicanthus melanospilos Japanese swallow angelreef safe lg Genicanthus melanospilos Japanese swallow angelmale lg Pomocanthus annularis Blue ring angel Juv. md Pomacanthus imperator Emperor angel Juv. md Pomocanthus semicirculatus Koran angel juv. md Pomacanthus sexstriatus Six banded angel Adult md Anthias Pseudanthias pleurotaenia Purple blotch anthiasmale md Pseudanthias randalli Randall's anthias md Pseudanthias randalli Randall's anthias male lg Pseudanthias truncatus Truncatus anthias md Pseudanthias tuka Purple queen anthias md Basslet Gramma linki Yellowlined basslet deepwater - RARE md Gramma loreto Royal gramma smd Gramma loreto Royal gramma md Gramma melacara Black cap basslet md Batfish Platax tiera Tiera batfish ml Blenny Acanthemblemaria aspera Rough head blenny xsm Acanthemblemaria maria Secretary blenny xsm Ecsenius bicolor Bicolor blennie md Ecsenius midas Gold midas blenny 1 left md Emblemaria pandionis Atlantic sailfin blenny md Meiacanthus grammistes Striped blenny md Meiacanthus smithi Smith's blenny md Ophioblennius atlanticus Redlip blenny md Parablennius marmoreus Horned seaweed blenny md Salarias fasciatus Sailfin blenny md Salarias fasciatus -

Coral Bleaching – a Review of the Causes and Consequences Chapter 4 a Reef Manager’S Guide to Coral Bleaching
CORAL BLEACHING – A REVIEW OF THE CAUSES AND CONSEQUENCES CHAPTER 4 A REEF MANAGER’S GUIDE TO CORAL BLEACHING 4. CORAL BLEACHING – A REVIEW OF THE CAUSES AND CONSEQUENCES The mass coral bleaching events that have occurred throughout the tropics over the last decade have provided unprecedented opportunity, and motivation, to study this phenomenon.As a result, knowledge about the causes and consequences of coral bleaching has increased substantially in recent years. This accumulating body of information is providing critical advances in our understanding and has generated new insights, which can assist reef managers to respond to the threat of coral bleaching. This section aims to provide a summary of recent developments in the science of coral bleaching, highlighting emerging knowledge and recent insights that are most relevant to reef managers. 4.1 What is coral bleaching? 4.1.1 The coral-algal symbiosis The great majority of corals live in a symbiotic relationship with zooxanthellae, a type of single-celled dinoflagellate algae.These microscopic algae live within the coral's tissues. Zooxanthellae produce energy-rich compounds through photosynthesis, providing a food source that is absorbed and used by the coral. In general, corals are highly dependent on © Kirsten Michalek-Wagner The zooxanthellae can be clearly this symbiotic relationship, receiving up to 90 per cent of seen as golden-coloured dots in their energy requirements in this way17. this close-up image of a coral polyp.The symbiotic relationship Bleaching is a stress response that results when the coral- with these tiny dinoflagellates enables corals to gain energy algae relationship breaks down. -

The Steinhart Aquarium
THE STEINHART AQUARIUM A VIEW FOR AND BY DOCENTS THE STEINHART AQUARIUM A FIELD GUIDE FOR AND BY DOCENTS ii A Docent & Guide View of the Steinhart Aquarium Species FOREWORD AND ACKNOWLEDGMENTS The Steinhart Aquarium, a central part of the California Academy of Sciences since 1923, two years ago opened a complex of exhibits as innovative and exciting as the institution that houses it. With the Steinhart’s spectacular return to Golden Gate Park, docents, who everyday share their passion and insight with the public, needed access to useful information specifically about the ever-growing and changing Aquarium collection of live animals. This Field Guide along with the photo IDs of the inhabitants of multispecies tanks hopes to fill an important part of that need. This digital guide, easy to update, is well suited to track the on-going diversification of Aquarium animals. Ideally, the Field Guide will be a resource improved and updated by information and suggestions from our Academy family—curators, staff, docents, guides and other volunteers, and all who love our finny, tentacled, slithering, gliding, flying, arboreal, aquatic, terrestrial denizens—all 38,000 of them. This is a book created by volunteers for volunteers; contributors and advisors were many and appreciated! Researchers and Writers: Maureen Aggeler, Ellen Barth, Roberta Borgonovo, Susan Crocker, Susana Conde, Pat Dal Porto, Steve Doherty, Arville Finacom, Ann Hardeman, Sandy Linder, Ted Olsson, Will Meecham, Alan Pabst, Owen Raven, Mary Roberts, Maggie Scott, Alice Settle, Elizabeth Shultz. Peter Schmidt earns a special star for the original conception of a field guide and for writing well over half of the entries, even more if the first two editions are counted. -

A Reef Manager's Guide to Coral Bleaching
A Reef Manager’s Guide to CORAL BLEACHING Paul Marshall and Heidi Schuttenberg The designation of geographical entities in this book, and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of GBRMPA, NOAA, IUCN or other participating organisations concerning the legal status of any country, territory or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. The views expressed in this publication do not necessarily reflect those of IUCN, US EPA or other participating organisations. This publication has been made possible in part by funding from the US National Oceanic and Atmospheric Administration and IUCN - The World Conservation Union. Published by: Great Barrier Reef Marine Park Authority,Townsville, Australia. Copyright: © 2006 Great Barrier Reef Marine Park Authority Reproduction of this publication for educational or other non-commercial purposes is authorised without prior written permission from the copyright holder provided the source is fully acknowledged. Reproduction of this publication for resale or other commercial purposes is prohibited without prior written permission of the copyright holder. Unless otherwise noted, photographs are copyright GBRMPA. National Library of Australia Cataloguing-in-Publication data: Marshall, Paul, 1969 A reef manager’s guide to coral bleaching. Bibliography. ISBN 1 876945 40 0 I. Coral reef conservation. 2. Coral reef ecology. 3. Coral reefs and islands. I. Schuttenberg, Heidi, 1973-II.Title. 639.972 Cover design and layout by: AECgroup Available from: Great Barrier Reef Marine Park Authority 2-68 Flinders St, PO Box 1379,Townsville, Qld 4810, Australia Tel: +61 7 4750 0700, Fax: +61 7 4772 6093 Email: [email protected] IUCN Publications Services Unit 219c Huntingdon Road, Cambridge CB3 0DL, United Kingdom Tel: +44 1223 277894, Fax: +44 1223 277175 E-mail: [email protected] www.iucn.org/bookstore A catalogue of IUCN publications is also available. -

Journal of Ichthyology and Aquatic Biology
aqua Journal of Ichthyology and Aquatic Biology Vol. 6 (3), February 2003 Aquapress ISSN 0945-9871 aqua - Journal of Ichthyology and Aquatic Biology Managing Editor: Scope and aims Heiko Bleher aqua is an international journal which publishes original Via G. Falcone 11, 27010 Miradolo Terme (PV), Italy scientific articles in the fields of systematics, taxonomy, Tel.: +39 0382 754707/08 - Fax: +39 0382 754129 bio geography, ethology, ecology, and general biology of e-mail: [email protected] fishes, amphibians, aquatic invertebrates, and plants. Papers on freshwater, brackish, and marine organisms Scientific Editor: will be considered. aqua is fully refereed and aims at publishing manuscripts within 2-4 months of acceptance. Dr. Walter Ivantsoff With the publi cation of aqua we are pursuing a new con- Senior Research Fellow, cept: In view of the importance of colour patterns in Department of Biological Sciences, species identi fication and animal ethology, authors are Macquarie University, N.S.W. 2109, Australia encouraged to submit colour illustrations as well as Tel. +61 2 9850 8167 - Fax +61 2 9869 8886 descriptions of coloration. It is our aim to provide e-mail: [email protected] the international scientific community with an efficiently published series meeting high scientific and technical Editorial Board: standards. Gerald R. Allen, I Dreyer Road Roleystone, W. A. Australia 6111 Call for papers George W. Barlow, Department of Integrative Biology, The editors welcome the submission of original manu- University of California, Berkeley, CA 94720-3140, scripts which should be sent directly to the scientific U.S.A. editor. Full length research papers and short notes will be considered for publication.