At Bimini, Bahamas
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Caribbean Reef Squid)
UWI The Online Guide to the Animals of Trinidad and Tobago Ecology Sepioteuthis sepioidea (Caribbean Reef Squid) Order: Teuthida (Squid) Class: Cephalopoda (Octopuses, Squid and Cuttlefish) Phylum: Mollusca (Molluscs) Fig. 1. Caribbean reef squid, Sepioteuthis sepioidea. [http://www.arkive.org/caribbean-reef-squid/sepioteuthis-sepioidea/image-G76785.html, downloaded 10 March 2016] TRAITS. The mantle (body mass) is wide and relatively flattened, with a length of 114mm in adult males and 120 mm in adult females (Moynihan and Rodaniche, 1982). A skeleton is absent but a cartilaginous layer is normally found beneath the surface of the mantle which enables movement (Mather et al., 2010).Two fins span the length of the lateral mantle margins (Fig. 1). The head is slightly pointed to its anterior end, with eight arms and two tentacles which encircle the mouth (Mather et al., 2010). Suckers are positioned along the inner region of arms and tentacle clubs. The mantle is fleshy when relaxed and the skin is very fragile (Moynihan and Rodaniche, 1982). The colour patterns of the skin can change periodically, due to the existence of light-reflective and iridescence-inducing cells (Mather, 2010). DISTRIBUTION. Distributed throughout the West Indian islands, including Trinidad and Tobago; widespread along the Central and South American coasts adjacent to the Caribbean Sea and also found in Bermuda and Florida (Moynihan and Rodaniche, 1982). UWI The Online Guide to the Animals of Trinidad and Tobago Ecology HABITAT AND ACTIVITY. Found in highly saline, clear waters of marine habitats at varying depths and distances from shoreline (Wood et al., 2008). The depth and habitat they are observed at depends on their growth stage (Mather et al., 2010). -

St. Kitts Final Report
ReefFix: An Integrated Coastal Zone Management (ICZM) Ecosystem Services Valuation and Capacity Building Project for the Caribbean ST. KITTS AND NEVIS FIRST DRAFT REPORT JUNE 2013 PREPARED BY PATRICK I. WILLIAMS CONSULTANT CLEVERLY HILL SANDY POINT ST. KITTS PHONE: 1 (869) 765-3988 E-MAIL: [email protected] 1 2 TABLE OF CONTENTS Page No. Table of Contents 3 List of Figures 6 List of Tables 6 Glossary of Terms 7 Acronyms 10 Executive Summary 12 Part 1: Situational analysis 15 1.1 Introduction 15 1.2 Physical attributes 16 1.2.1 Location 16 1.2.2 Area 16 1.2.3 Physical landscape 16 1.2.4 Coastal zone management 17 1.2.5 Vulnerability of coastal transportation system 19 1.2.6 Climate 19 1.3 Socio-economic context 20 1.3.1 Population 20 1.3.2 General economy 20 1.3.3 Poverty 22 1.4 Policy frameworks of relevance to marine resource protection and management in St. Kitts and Nevis 23 1.4.1 National Environmental Action Plan (NEAP) 23 1.4.2 National Physical Development Plan (2006) 23 1.4.3 National Environmental Management Strategy (NEMS) 23 1.4.4 National Biodiversity Strategy and Action Plan (NABSAP) 26 1.4.5 Medium Term Economic Strategy Paper (MTESP) 26 1.5 Legislative instruments of relevance to marine protection and management in St. Kitts and Nevis 27 1.5.1 Development Control and Planning Act (DCPA), 2000 27 1.5.2 National Conservation and Environmental Protection Act (NCEPA), 1987 27 1.5.3 Public Health Act (1969) 28 1.5.4 Solid Waste Management Corporation Act (1996) 29 1.5.5 Water Courses and Water Works Ordinance (Cap. -

Belonidae Bonaparte 1832 Needlefishes
ISSN 1545-150X California Academy of Sciences A N N O T A T E D C H E C K L I S T S O F F I S H E S Number 16 September 2003 Family Belonidae Bonaparte 1832 needlefishes By Bruce B. Collette National Marine Fisheries Service Systematics Laboratory National Museum of Natural History, Washington, DC 20560–0153, U.S.A. email: [email protected] Needlefishes are a relatively small family of beloniform fishes (Rosen and Parenti 1981 [ref. 5538], Collette et al. 1984 [ref. 11422]) that differ from other members of the order in having both the upper and the lower jaws extended into long beaks filled with sharp teeth (except in the neotenic Belonion), the third pair of upper pharyngeal bones separate, scales on the body relatively small, and no finlets following the dorsal and anal fins. The nostrils lie in a pit anterior to the eyes. There are no spines in the fins. The dorsal fin, with 11–43 rays, and anal fin, with 12–39 rays, are posterior in position; the pelvic fins, with 6 soft rays, are located in an abdominal position; and the pectoral fins are short, with 5–15 rays. The lateral line runs down from the pectoral fin origin and then along the ventral margin of the body. The scales are small, cycloid, and easily detached. Precaudal vertebrae number 33–65, caudal vertebrae 19–41, and total verte- brae 52–97. Some freshwater needlefishes reach only 6 or 7 cm (2.5 or 2.75 in) in total length while some marine species may attain 2 m (6.5 ft). -

Cruise Report W-48 Scientific Activities Undertaken Aboard R/V Westward Woods Hole
Cruise Report W-48 Scientific Activities Undertaken Aboard R/V Westward Woods Hole - St. Thomas 10 October - 21 November 1979 ff/lh Westward (R.Long) • Sea Education Association - Woods Hole, Massachusetts " CRUISE REPORT W-48 Scientific Activities Woods Hole - Antigua - St. Lucia - Bequia - St. Thomas 10 October 1979 - 21 November 1979 R/V Westward Sea Education Association ',,, Woods Hole, Massachusetts .. SHIPBOARD DRAFT .. ----------------------- - ( PREFACE This Cruise Report is written in an attempt to accomplish two objectives. Firstly, and more importantly, it presents a brief outline of the scientific research completed aboard R/V Westward during W-48. Reports of the status of on-going projects and of the traditional academic program are presented. In addition, abstracts from the research projects of each student are included. Secondly, for those of us that participated, it represents the product of our efforts and contains a record of other events that were an important part of the trip, in particular the activities during port stops. Once again, lowe special thanks to Abby Ames, who was in charge of the shipboard laboratory, and upon whom I was able to depend through out the cruise. Her effectiveness and perseverance under the difficult working conditions at sea, and her cheerful attitude and enthusiasm were greatly appreciated by us all. Rob Nawojchik, who participated as an Assistant Scientist, added a new field of interest to the cruise with his vast knowledge of ichthyology. The energy with which he pursued his interest and his enthusiasm for the subject, set an example for us all. Two visiting scholars participated in different legs of this cruise. -

Checklists of Crustacea Decapoda from the Canary and Cape Verde Islands, with an Assessment of Macaronesian and Cape Verde Biogeographic Marine Ecoregions
Zootaxa 4413 (3): 401–448 ISSN 1175-5326 (print edition) http://www.mapress.com/j/zt/ Article ZOOTAXA Copyright © 2018 Magnolia Press ISSN 1175-5334 (online edition) https://doi.org/10.11646/zootaxa.4413.3.1 http://zoobank.org/urn:lsid:zoobank.org:pub:2DF9255A-7C42-42DA-9F48-2BAA6DCEED7E Checklists of Crustacea Decapoda from the Canary and Cape Verde Islands, with an assessment of Macaronesian and Cape Verde biogeographic marine ecoregions JOSÉ A. GONZÁLEZ University of Las Palmas de Gran Canaria, i-UNAT, Campus de Tafira, 35017 Las Palmas de Gran Canaria, Spain. E-mail: [email protected]. ORCID iD: 0000-0001-8584-6731. Abstract The complete list of Canarian marine decapods (last update by González & Quiles 2003, popular book) currently com- prises 374 species/subspecies, grouped in 198 genera and 82 families; whereas the Cape Verdean marine decapods (now fully listed for the first time) are represented by 343 species/subspecies with 201 genera and 80 families. Due to changing environmental conditions, in the last decades many subtropical/tropical taxa have reached the coasts of the Canary Islands. Comparing the carcinofaunal composition and their biogeographic components between the Canary and Cape Verde ar- chipelagos would aid in: validating the appropriateness in separating both archipelagos into different ecoregions (Spalding et al. 2007), and understanding faunal movements between areas of benthic habitat. The consistency of both ecoregions is here compared and validated by assembling their decapod crustacean checklists, analysing their taxa composition, gath- ering their bathymetric data, and comparing their biogeographic patterns. Four main evidences (i.e. different taxa; diver- gent taxa composition; different composition of biogeographic patterns; different endemicity rates) support that separation, especially in coastal benthic decapods; and these parametres combined would be used as a valuable tool at comparing biotas from oceanic archipelagos. -

Part I. an Annotated Checklist of Extant Brachyuran Crabs of the World
THE RAFFLES BULLETIN OF ZOOLOGY 2008 17: 1–286 Date of Publication: 31 Jan.2008 © National University of Singapore SYSTEMA BRACHYURORUM: PART I. AN ANNOTATED CHECKLIST OF EXTANT BRACHYURAN CRABS OF THE WORLD Peter K. L. Ng Raffles Museum of Biodiversity Research, Department of Biological Sciences, National University of Singapore, Kent Ridge, Singapore 119260, Republic of Singapore Email: [email protected] Danièle Guinot Muséum national d'Histoire naturelle, Département Milieux et peuplements aquatiques, 61 rue Buffon, 75005 Paris, France Email: [email protected] Peter J. F. Davie Queensland Museum, PO Box 3300, South Brisbane, Queensland, Australia Email: [email protected] ABSTRACT. – An annotated checklist of the extant brachyuran crabs of the world is presented for the first time. Over 10,500 names are treated including 6,793 valid species and subspecies (with 1,907 primary synonyms), 1,271 genera and subgenera (with 393 primary synonyms), 93 families and 38 superfamilies. Nomenclatural and taxonomic problems are reviewed in detail, and many resolved. Detailed notes and references are provided where necessary. The constitution of a large number of families and superfamilies is discussed in detail, with the positions of some taxa rearranged in an attempt to form a stable base for future taxonomic studies. This is the first time the nomenclature of any large group of decapod crustaceans has been examined in such detail. KEY WORDS. – Annotated checklist, crabs of the world, Brachyura, systematics, nomenclature. CONTENTS Preamble .................................................................................. 3 Family Cymonomidae .......................................... 32 Caveats and acknowledgements ............................................... 5 Family Phyllotymolinidae .................................... 32 Introduction .............................................................................. 6 Superfamily DROMIOIDEA ..................................... 33 The higher classification of the Brachyura ........................ -

Lac Bay: Then and Now… a Historical Interpretation of Environmental Change During the 1900S
Lac Bay: Then and Now… A Historical Interpretation of Environmental Change During the 1900s A Site Characterization of Lac Bay for Resource Managers and Naturalists prepared for Bonaire Marine Park by Environics, N.V. Cynthia E. Lott, Environmental Consultant Final Edition October 2001 Lac Bay Then and Now A Historical Interpretation of Environmental Change During the 1900s A Site Characterization of Lac Bay For Resource Managers and Naturalists By Cynthia E. Lott Environmental Consultant Bonaire, Netherlands Antilles Final Edition October 2001 Copyright 2001 All rights reserved Title Page Map Credits: Top: Year 1866, Year 1911 (Hummelinck en Roos 1969); Middle: Year 1949, Year 1967 (Hummelinck en Roos 1969); Bottom left: Year 1961 (Hummelinck en Roos 1969); Bottom right: Year 1998 (Wing Group, N.V. 1998/Dec.) This Work is dedicated… In the fondest memory of My beloved friend, Dick Harrison, who encouraged me to believe in myself. God Bless Us Every One…. Lac Bay: Then and Now…A Historical Interpretation of Environmental Change During the 1900s A Site Characterization for Resource Managers and Naturalists Foreword by Boi Antoin, Editor of EXTRA, Bonaire's Daily Newspaper, and respected local historian LAC No tin duda ku Lac ta un sitio, un bahia, ku nos mester trata na protehá i konservá na tur manera. E historia, kultura i naturalesa di Lac hamas por bai pèrdí. Asina importante e lugá aki tabata I ta pa Boneiru. Despues di investigashonnan arkeológiko i ekológiko ku a tuma lugá di Lac, tabata nesesario, na mi konsepto, pa a bini ku un investigashon mas profundo i sientífiko di e área bunita aki. -

Checklist of Philippine Chondrichthyes
CSIRO MARINE LABORATORIES Report 243 CHECKLIST OF PHILIPPINE CHONDRICHTHYES Compagno, L.J.V., Last, P.R., Stevens, J.D., and Alava, M.N.R. May 2005 CSIRO MARINE LABORATORIES Report 243 CHECKLIST OF PHILIPPINE CHONDRICHTHYES Compagno, L.J.V., Last, P.R., Stevens, J.D., and Alava, M.N.R. May 2005 Checklist of Philippine chondrichthyes. Bibliography. ISBN 1 876996 95 1. 1. Chondrichthyes - Philippines. 2. Sharks - Philippines. 3. Stingrays - Philippines. I. Compagno, Leonard Joseph Victor. II. CSIRO. Marine Laboratories. (Series : Report (CSIRO. Marine Laboratories) ; 243). 597.309599 1 CHECKLIST OF PHILIPPINE CHONDRICHTHYES Compagno, L.J.V.1, Last, P.R.2, Stevens, J.D.2, and Alava, M.N.R.3 1 Shark Research Center, South African Museum, Iziko–Museums of Cape Town, PO Box 61, Cape Town, 8000, South Africa 2 CSIRO Marine Research, GPO Box 1538, Hobart, Tasmania, 7001, Australia 3 Species Conservation Program, WWF-Phils., Teachers Village, Central Diliman, Quezon City 1101, Philippines (former address) ABSTRACT Since the first publication on Philippines fishes in 1706, naturalists and ichthyologists have attempted to define and describe the diversity of this rich and biogeographically important fauna. The emphasis has been on fishes generally but these studies have also contributed greatly to our knowledge of chondrichthyans in the region, as well as across the broader Indo–West Pacific. An annotated checklist of cartilaginous fishes of the Philippines is compiled based on historical information and new data. A Taiwanese deepwater trawl survey off Luzon in 1995 produced specimens of 15 species including 12 new records for the Philippines and a few species new to science. -

Etyfish Orectolobifo
ORECTOLOBIFORMES (Carpet Sharks) · 1 The ETYFish Project © Christopher Scharpf and Kenneth J. Lazara COMMENTS: v. 7.0 - 15 Oct. 2019 Order ORECTOLOBIFORMES Carpet Sharks 7 families · 13 genera · 45 species Family PARASCYLLIIDAE Collared Carpet Sharks 2 genera · 8 species Cirrhoscyllium Smith & Radcliffe 1913 cirrus, curl or tendril, referring to barbels on throat; skylion, Greek for dogfish or small shark, probably from skyllo, to tear or mangle Cirrhoscyllium expolitum Smith & Radcliffe 1913 varnished, referring to how the shark’s body, when dry, “glistens as though varnished, owing to the peculiar character of the dermal denticles” Cirrhoscyllium formosanum Teng 1959 -anum, adjectival suffix: referring to distribution off the coast of Formosa (Taiwan) Cirrhoscyllium japonicum Kamohara 1943 Japanese, known only from Mimase, Shikoku, Japan Parascyllium Gill 1862 para-, near, i.e., related to Scylliorhinus (now in Scyliorhinidae); skylion, Greek for dogfish or small shark, probably from skyllo, to tear or mangle Parascyllium collare Ramsay & Ogilby 1888 collar, referring to prominent dark and unspotted collar around gills Parascyllium elongatum Last & Stevens 2008 prolonged, referring to distinctive, elongate body shape Parascyllium ferrugineum McCulloch 1911 rust-colored, referring to dark brown spots on sides and fins Parascyllium sparsimaculatum Goto & Last 2002 sparsi, sparse; maculatum, spotted, referring to relatively larger (and hence fewer) spots than congeners Parascyllium variolatum (Duméril 1853) spotted, referring to white -
![Parascylliidae Gill, 1862 - Carpetsharks, Collared Carpetsharks [=Parascyllinae, Cirrhoscylliidae] Notes: Parascylliinae Gill, 1862P:408 [Ref](https://docslib.b-cdn.net/cover/1623/parascylliidae-gill-1862-carpetsharks-collared-carpetsharks-parascyllinae-cirrhoscylliidae-notes-parascylliinae-gill-1862p-408-ref-501623.webp)
Parascylliidae Gill, 1862 - Carpetsharks, Collared Carpetsharks [=Parascyllinae, Cirrhoscylliidae] Notes: Parascylliinae Gill, 1862P:408 [Ref
FAMILY Parascylliidae Gill, 1862 - carpetsharks, collared carpetsharks [=Parascyllinae, Cirrhoscylliidae] Notes: Parascylliinae Gill, 1862p:408 [ref. 1783] (subfamily) Parascyllium [family name sometimes seen as Parascyllidae] Cirrhoscylliidae Applegate, 1974:749 [ref. 31930] (family) Cirrhoscyllium GENUS Cirrhoscyllium Smith & Radcliffe, in Smith, 1913 - carpetsharks [=Cirrhoscyllium Smith [H. M.] & Radcliffe [L.], in Smith, 1913:568, Zev Whitley [G. P.], 1927:289] Notes: [ref. 4043]. Neut. Cirrhoscyllium expolitum Smith & Radcliffe, 1913. Type by original designation (also monotypic). Zev Whitley 1927 is an unneeded replacement. •Valid as Cirrhoscyllium Smith & Radcliffe, 1913 -- (Compagno 1984:167 [ref. 6474], Nakaya & Shirai in Masuda et al. 1984:8 [ref. 6441], Goto et al. 1994:167 [ref. 22834], Goto & Nakaya 1996:200 [ref. 22429], Compagno 1999:476 [ref. 25589], Goto 2001:80 [ref. 25912], Compagno 2001:132 [ref. 26323], Compagno et al. 2005:19 [ref. 29145]). Current status: Valid as Cirrhoscyllium Smith & Radcliffe, 1913. Parascylliidae. (Zev) [ref. 4662]. Masc. Cirrhoscyllium expolitum Smith & Radcliffe, 1913. Type by being a replacement name. Unneeded replacement for Cirrhoscyllium Smith & Radcliffe, 1913, not preoccupied by Cirriscyllium Ogilby, 1908 in same family. Treated as masculine (Art. 30.2.4). •Objective synonym of Cirrhoscyllium Smith & Radcliffe, 1913 -- (Compagno 1984:167 [ref. 6474], Goto & Nakaya 1996:200 [ref. 22429], Goto 2001:80 [ref. 25912]). Current status: Synonym of Cirrhoscyllium Smith & Radcliffe, 1913. Parascylliidae. Species Cirrhoscyllium expolitum Smith & Radcliffe, in Smith, 1913 - barbelthroat carpetshark [=Cirrhoscyllium expolitum Smith [H. M.] & Radcliffe [L.], in Smith, 1913:568, Pl. 45; Figs. 1, 2] Notes: [Proceedings of the United States National Museum v. 45 (no. 1997); ref. 4043] South China Sea between northern Luzon Island and China, 21°33'N, 118 [or 116]°13'E, Albatross station 5310, depth 100 fathoms. -

Life History, Mating Behavior, and Multiple Paternity in Octopus
LIFE HISTORY, MATING BEHAVIOR, AND MULTIPLE PATERNITY IN OCTOPUS OLIVERI (BERRY, 1914) (CEPHALOPODA: OCTOPODIDAE) A DISSERTATION SUBMITTED TO THE GRADUATE DIVISION OF THE UNIVERSITY OF HAWAI´I AT MĀNOA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN ZOOLOGY DECEMBER 2014 By Heather Anne Ylitalo-Ward Dissertation Committee: Les Watling, Chairperson Rob Toonen James Wood Tom Oliver Jeff Drazen Chuck Birkeland Keywords: Cephalopod, Octopus, Sexual Selection, Multiple Paternity, Mating DEDICATION To my family, I would not have been able to do this without your unending support and love. Thank you for always believing in me. ii ACKNOWLEDGMENTS I would like to thank all of the people who helped me collect the specimens for this study, braving the rocks and the waves in the middle of the night: Leigh Ann Boswell, Shannon Evers, and Steffiny Nelson, you were the hard core tako hunters. I am eternally grateful that you sacrificed your evenings to the octopus gods. Also, thank you to David Harrington (best bucket boy), Bert Tanigutchi, Melanie Hutchinson, Christine Ambrosino, Mark Royer, Chelsea Szydlowski, Ily Iglesias, Katherine Livins, James Wood, Seth Ylitalo-Ward, Jessica Watts, and Steven Zubler. This dissertation would not have happened without the support of my wonderful advisor, Dr. Les Watling. Even though I know he wanted me to study a different kind of “octo” (octocoral), I am so thankful he let me follow my foolish passion for cephalopod sexual selection. Also, he provided me with the opportunity to ride in a submersible, which was one of the most magical moments of my graduate career. -
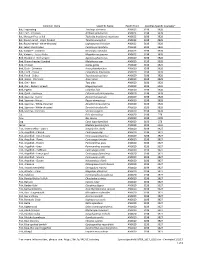
*For More Information, Please See
Common Name Scientific Name Health Point Specifies-Specific Course(s)* Bat, Frog-eating Trachops cirrhosus AN0023 3198 3928 Bat, Fruit - Jamaican Artibeus jamaicensis AN0023 3198 3928 Bat, Mexican Free-tailed Tadarida brasiliensis mexicana AN0023 3198 3928 Bat, Round-eared - stripe-headed Tonatia saurophila AN0023 3198 3928 Bat, Round-eared - white-throated Lophostoma silvicolum AN0023 3198 3928 Bat, Seba's short-tailed Carollia perspicillata AN0023 3198 3928 Bat, Vampire - Common Desmodus rotundus AN0023 3198 3928 Bat, Vampire - Lesser False Megaderma spasma AN0023 3198 3928 Bird, Blackbird - Red-winged Agelaius phoeniceus AN0020 3198 3928 Bird, Brown-headed Cowbird Molothurus ater AN0020 3198 3928 Bird, Chicken Gallus gallus AN0020 3198 3529 Bird, Duck - Domestic Anas platyrhynchos AN0020 3198 3928 Bird, Finch - House Carpodacus mexicanus AN0020 3198 3928 Bird, Finch - Zebra Taeniopygia guttata AN0020 3198 3928 Bird, Goose - Domestic Anser anser AN0020 3198 3928 Bird, Owl - Barn Tyto alba AN0020 3198 3928 Bird, Owl - Eastern Screech Megascops asio AN0020 3198 3928 Bird, Pigeon Columba livia AN0020 3198 3928 Bird, Quail - Japanese Coturnix coturnix japonica AN0020 3198 3928 Bird, Sparrow - Harris' Zonotrichia querula AN0020 3198 3928 Bird, Sparrow - House Passer domesticus AN0020 3198 3928 Bird, Sparrow - White-crowned Zonotrichia leucophrys AN0020 3198 3928 Bird, Sparrow - White-throated Zonotrichia albicollis AN0020 3198 3928 Bird, Starling - Common Sturnus vulgaris AN0020 3198 3928 Cat Felis domesticus AN0020 3198 279 Cow Bos taurus