Bioactive Components of Vigna Species: Current Prospective
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Prespective Study of Clitoria Ternatia and Its Pharmacological Importance
High Technology Letters ISSN NO : 1006-6748 A PRESPECTIVE STUDY OF CLITORIA TERNATIA AND ITS PHARMACOLOGICAL IMPORTANCE S. GEJALAKSHMI *1, N. HARIKRISHNAN FACULTY OF PHARMACY, DR.M.G.R. EDUCATIONAL AND RESEARCH INSTITUTE, VELAPPANCHAVADI, CHENNAI-77 Abstract: Medicinal herbs and aromatic plants have been extensively used for the past few decades due to its potency and minimal side effects. By observing the medicinal importance of the climbing herb Clitoria ternatea (CT)of Fabeacea family and commonly known as Butterfly pea and Shankpushpi has been taken up due to its high medicinal value due to its wide range of use over decade as memory enhancer,antidepressant,anticonvulsant,transquilizers and sedative agent.A series of secondary metabolite including triterpenoids,flavone glycosides,anthocyanins and i steroids has been isolated from CT extracts.CT plant has wide range of pharmacological activity such as antimicrobial,antipyretic,diuretic,local anaesthetic.CT has been used for several diseases due to availability of several active constituents like alkaloids,flavanoids,saponins,tannins,carbohydrates .This review is an platform to explore the phytochemical investigation and pharmacological importance of CT,which have been practiced in traditional system of medicine and its future potential prespectives in view of innumerable therapeutic importance on this well-known twinning climber. Key words: Shankpushpi,phytochemical,antibacterial,anti- fungal, anti-cancer Introduction: Herbal drugs has an impact for curing disorders. The medicinal herbs are rich in various phytochemical constituents which has been found for traditional system of medicines. In the present reveiw focused on the traditional importance of clitoria ternatea. (CT).It is perennial twinning herb. It is a member of fabiaecea family and it has various synonym like blue pea. -

Alien Introgression Studies Involving Vigna Mungo X Vigna Umbellata Hybridization
Int.J.Curr.Microbiol.App.Sci (2020) 9(2): 268-276 International Journal of Current Microbiology and Applied Sciences ISSN: 2319-7706 Volume 9 Number 2 (2020) Journal homepage: http://www.ijcmas.com Original Research Article https://doi.org/10.20546/ijcmas.2020.902.034 Alien Introgression Studies Involving Vigna mungo x Vigna umbellata Hybridization Shayla Bindra1*, R.K. Mittal2, V.K. Sood2 and H.K. Chaudhary2 1Department of Plant Breeding & Genetics, Punjab Agricultural University, Ludhiana, Punjab, India 2Department of Crop Improvement, CSK Himachal Pradesh Agricultural University, Palampur, Himachal Pradesh, India *Corresponding author ABSTRACT Urdbean has narrow genetic base due to common ancestry of various K e yw or ds superior genotypes, poor plant type and genetic vulnerability to biotic and abiotic stresses. The underutilized related specie, V. umbellata (ricebean), Alien introgression, Urdbean, Ricebean, nutritive and resistant to most of the fungal pathogens, is speculated to be hybridization potential donor. Therefore, V. mungo and V. umbellata were crossed with a view to introgress alien genes. On inter-specific hybridization, the presence Article Info of pre-fertilization barriers and post-fertilization barriers were observed. Accepted: As, hybrids exhibited reduced germination and sterility. The frequency of 05 January 2020 hybridization, radicle and plant production percentage among three cross Available Online: combinations revealed genotype specific response between both the 10 February 2020 species. Introduction ancestry, poor plant type, vulnerability to biotic and abiotic stresses and its cultivation in The grain legume, urdbean (Vigna mungo L. marginal land and harsh environmental Hepper) also known as blackgram, black conditions. It is susceptible to various fungal lentil, mungo bean, black matpe bean. -

Butterfly Pea (Clitoria Ternatea) | Feedipedia
Butterfly pea (Clitoria ternatea) | Feedipedia Animal feed resources Feedipedia information system Home About Feedipedia Team Partners Get involved Contact us Butterfly pea (Clitoria ternatea) Automatic translation Description Nutritional aspects Nutritional tables References Sélectionner une langue ▼ Click on the "Nutritional aspects" tab for recommendations for ruminants, pigs, poultry, rabbits, horses, fish and crustaceans Feed categories All feeds Forage plants Cereal and grass forages Legume forages Forage trees Aquatic plants Common names Other forage plants Plant products/by-products Butterfly pea, blue pea, kordofan pea, cordofan pea, Asian pigeonwings [English]; pois bleu [French]; clitoria azul, azulejo, Cereal grains and by-products papito, zapatico de la reina, zapotillo, conchita azul, campanilla, bandera, choroque, lupita, pito de parra, bejuco de conchitas Legume seeds and by-products [Spanish]; cunhã, Fula criqua [Portuguese]; kittelbloem [Dutch]; Blaue Klitorie [German]; tembang telang [Indonesian]; Bunga Oil plants and by-products telang [Malay]; Mavi Kelebek Sarmaşığı [Turkish]; Chi Đậu biếc [Vietnamese]; [Bengali]; 蝶豆 [Chinese]; Fruits and by-products [Hindi]; [Malayalam]; [Marathi]; [Tamul]; [Telugu]; Roots, tubers and by-products ดอกอญชั นั [Thai] Sugar processing by-products Plant oils and fats Species Other plant by-products Feeds of animal origin Clitoria ternatea L. [Fabaceae] Animal by-products Dairy products/by-products Synonyms Animal fats and oils Insects Clitoria albiflora Mattei; Clitoria bracteata Poir.; Clitoria mearnsii De Wild.; Clitoria tanganicensis Micheli; Clitoria zanzibarensis Other feeds Vatke Minerals Other products Feed categories Legume forages Legume seeds and by-products Forage plants Latin names Plant and animal families Related feed(s) Plant and animal species Description Resources The butterfly pea (Clitoria ternatea L.) is a vigorous, trailing, scrambling or climbing tropical legume. -

Impact of Cobalt on Germination and Seedling Growth of Eleusine Coracana L
Global Journal of Molecular Sciences 3 (1): 18-20, 2008 ISSN 1990-9241 © IDOSI Publications, 2008 Impact of Cobalt on Germination and Seedling Growth of Eleusine coracana L. And Oryza sativa L. Under Hydroponic Culture 1Kaliyamoorthy Jayakumar, 1,2Cheruth Abdul Jaleel and 3,4M.M. Azooz 1Stress Physiology Lab, Department of Botany, Annamalai University, Annamalainagar 608 002, Tamilnadu, India 2DMJM International (AECOM Middle East Ltd.), Consultant of Gardens Sector Projects, Alain Municipality and Eastern Emirates, P.O. Box 1419, Al-Ain, Abu Dhabi, United Arab Emirates 3Department of Botany, Faculty of Science, South Valley University, 83523 Qena, Egypt 4Department of Biology, Faculty of Science, King Faisal University, P.O. Box: 380, Al-Hassa 31982, Saudi Arabia Abstract: Germination studies were conducted in ragi (Eleusine coracana L.) and paddy (Oryza sativa L.) inorder to find out the impact of soil cobalt level on germination and seedling vigour. The seeds of ragi and paddy were germinated with six concentrations of cobalt chloride solution ranging from 5-100 mg/l in hydroponic condition upto 8 days. The germination was found increased significantly under low level of cobalt with decreased in germination and reduction in the length of radical and plumule were observed in seeds of ragi and paddy. Vigour index, tolerance index and dry weight of root and shoot of the seedlings increased at low level of cobalt treatments and decreased with increase in cobalt concentrations. However, the germination percentage of ragi and paddy seeds showed a significant difference with cobalt treatment. Key words: Cobalt, Ragi, Paddy, Germination, Seedling, Vigour, Hydroponics INTRODUCTION Coimbatore and seeds of paddy (Oryza sativa L.) cv ADT-43 were obtained from Tamilnadu Rice Research The presence of heavy metals in the environment is Institute, Aduthurai. -

Evolvulus Alsinoides (Convolvulaceae): an American Herb in the Old World Daniel F
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues. Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited. In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright Author's personal copy Available online at www.sciencedirect.com Journal of Ethnopharmacology 117 (2008) 185–198 Review Evolvulus alsinoides (Convolvulaceae): An American herb in the Old World Daniel F. Austin Arizona-Sonora Desert Museum, 2021 North Kinney Road, Tucson, AZ 85743, USA Received 23 October 2007; received in revised form 28 January 2008; accepted 29 January 2008 Available online 12 February 2008 Abstract People in the Indian region often apply shankhapushpi and vishnukranti, two Sanskrit-based common names, to Evolvulus alsinoides. These are pre-European names that are applied to a medicinal American species transported into the area. The period of introduction is uncertain, but probably took place in the 1500s or 1600s. Examination of relationships of Evolvulus alsinoides, geographic distribution, its names in Asia, medical uses, and chemical and laboratory analysis indicates that the alien plant was adopted, given an ancient Indian name, and incorporated into some Old World pharmacopoeias. The herb apparently was included in medicines because it not only reminded people of certain aspects of their gods and goddesses, but also because the chemicals it contained were useful against some maladies. -

Morphological and Agronomic Traits Variations for Mungbean Variety Selection and Improvement in Uganda
African Crop Science Journal, Vol. 22, No. 2, pp. 123 - 136 ISSN 1021-9730/2014 $4.00 Printed in Uganda. All rights reserved © 2014, African Crop Science Society MORPHOLOGICAL AND AGRONOMIC TRAITS VARIATIONS FOR MUNGBEAN VARIETY SELECTION AND IMPROVEMENT IN UGANDA A. WANIALE, N. WANYERA1 and H. TALWANA School of Agricultural and Environmental Sciences, Makerere University, P. O. Box 7062, Kampala, Uganda 1National Semi-Arid Resources Research Institute, National Agricultural Research Organization, P. O. Box Soroti, Uganda Corresponding author: [email protected] (Received 18 December, 2013; accepted 5 May, 2014) ABSTRACT Mungbean (Vigna radiata L. Wilczek), is a pulse species that is widely cultivated in sub-tropical and tropical regions of the world. Unfortunately, the yield of mungbean in Uganda is very low mainly due to inherent genotype failures and losses due to pests and diseases. To achieve a gain in yield through breeding requires collection, characterisation, and evaluation of germplasm, as the first step in identifying genotypes with the desired characteristics. The objective of this study was to describe the nature and extent of genotypic variation among mungbean collections for a range of traits of potential agronomic and adaptive interests in Uganda. A total of 35 mungbean accessions acquired mainly from the World Vegetable Centre (AVRDC) in Taiwan, two local ricebean (Vigna umbellata (Thunb.) Ohwi and Ohashi) and one local blackgram genotype (Vigna mungo) were evaluated for several diverse traits for two cropping seasons at two different locations in Uganda. Genotype by environment interaction (GEI) was significant (P < 0.001) for all the traits, indicating inconsistent performance by some genotypes across two locations and two seasons. -

Genetic Analysis of Yield and Yield Attributing Characters of Blackgram (Vigna Mungo (L.) Hepper) During Summer Season in Gangetic Alluvial Soil of West Bengal
Electronic Journal of Plant Breeding, 10 (3): 1210 - 1217(Sep 2019) DOI: 10.5958/0975-928X.2019.00153.4 Genetic analysis ISSN 0975-928X of yield and yield attributing characters of blackgram (Vigna mungo (L.) Hepp er) during summer season in gangetic alluvial soil of West Bengal Ram Babu Raman, Nandan Manna, Sudipta Patra and Narayan Chandra Sahu ISSN: 0975-928X Volume: 10 Number:3 EJPB (2019) 10(3):1210-1217 DOI:10.5958/0975-928X.2019.00153.4 1209 https://ejplantbreeding.org Electronic Journal of Plant Breeding, 10 (3): 1210 - 1217(Sep 2019) DOI: 10.5958/0975-928X.2019.00153.4 ISSN 0975-928X Research Article Genetic analysis of yield and yield attributing characters of blackgram (Vigna mungo (L.) Hepper) during summer season in gangetic alluvial soil of West Bengal Ram Babu Raman*1, Nandan Manna2, Sudipta Patra3 and Narayan Chandra Sahu4 1,4Sasya Shyamala Krishi Vigyan Kendra, Ramakrishna Mission Vivekananda Educational and Research Institute (RKMVERI), Arapanch, Sonarpur, Kolkata-700150 2,3Integrated Rural Development and Management (IRDM) School of Agriculture and Rural Development, Ramakrishna Mission Vivekananda Educational and Research Institute (RKMVERI), Ramakrishna Mission Ashrama, Narendrapur Kolkata- 700103 E-Mail: [email protected] (Received: 04 Oct 2018; Revised: 17 Apr 2019; Accepted: 26 Jul 2019) Abstract The experiment was laid out with twenty genotypes and three replications at Instructional Farm of Ramakrishna Mission Vivekananda Educational and Research Institute, Narendrapur, Kolkata, West Bengal. Among the genotypes RSU-03 showed significantly superior for number of branches per plant (9.07), number of pods per plant (26.27), 100 seed weight (5.15 g) and yield per plant (11.53 g). -

Healthy Food Traditions of Asia: Exploratory Case Studies From
Harmayani et al. Journal of Ethnic Foods (2019) 6:1 Journal of Ethnic Foods https://doi.org/10.1186/s42779-019-0002-x ORIGINALARTICLE Open Access Healthy food traditions of Asia: exploratory case studies from Indonesia, Thailand, Malaysia, and Nepal Eni Harmayani1, Anil Kumar Anal2, Santad Wichienchot3, Rajeev Bhat4, Murdijati Gardjito1, Umar Santoso1, Sunisa Siripongvutikorn5, Jindaporn Puripaatanavong6 and Unnikrishnan Payyappallimana7* Abstract Asia represents rich traditional dietary diversity. The rapid diet transition in the region is leading to a high prevalence of non-communicable diseases. The aim of this exploratory study was to document traditional foods and beverages and associated traditional knowledge that have potential positive health impacts, from selected countries in the region. The study also focused on identifying their importance in the prevention and management of lifestyle-related diseases and nutritional deficiencies as well as for the improvement of the overall health and wellbeing. This was conducted in selected locations in Indonesia, Thailand, Malaysia and Nepal through a qualitative method with a pre-tested documentation format. Through a detailed documentation of their health benefits, the study tries to highlight the significance of traditional foods in public health as well as their relevance to local market economies towards sustainable production and consumption and sustainable community livelihoods. Keywords: Traditional foods, Ethnic recipes, Asian health food traditions, Cultural dietary diversity, Indonesia, Thailand, Malaysia and Nepal Introduction Due to the dynamic adaptations to local biocultural con- Asia represents vast geographic, socioeconomic, bio- texts and refinement over generations through empirical logical, and cultural diversity. This is also reflected in the observations, they assume to have positive health impacts dietary diversity of traditional foods. -

Collection and Conservation of Leguminous Crops and Their Wild Relatives in Cambodia, 2013
〔AREIPGR Vol. 30 : 109 ~ 143 ,2014〕 Original Paper Collection and Conservation of Leguminous Crops and Their Wild Relatives in Cambodia, 2013 Yu TAKAHASHI 1), 2), Uong PEOU 3), Seang LAY HENG 3), Ty CHANNA 3), Ouk MAKARA 3) and Norihiko TOMOOKA 1) 1) Genetic Resources Center, National Institute of Agrobiological Sciences, Kannondai 2-1-2, Tsukuba, Ibaraki 305-8602, Japan 2) Research Fellow of the Japan Society for the Promotion of Science 3) Cambodian Agricultural Research and Development Institute, National Road 3, Prateahlang, Dangkor, P.O Box 01, Phnom Penh, Cambodia Corresponding author : N. TOMOOKA (e-mail : [email protected]). Summary We have conducted a field survey on the leguminous plants in Cambodia from 18th to 27th November, 2013. A total of 74 accessions were collected, including Vigna minima (Roxb.) Ohwi & Ohashi, Vigna umbellata (Thunb.) Ohwi & Ohashi, Vigna radiata (L.) Wilczek, Vigna reflexo-pilosa Hayata, Vigna unguiculata (L.) Walp., Phaseolus vulgaris L. and Glycine max (L.) Merr.. The seeds had been conserved in the Cambodian Agricultural Research and Development Institute (CARDI) genebank, and the subset was transferred to the National Institute of Agrobiological Sciences (NIAS) genebank. We plan to multiply the seeds and evaluate their growth traits in NIAS, Japan. KEY WORDS : Cambodia, Legume, Vigna, Phaseolus vulgaris, Glycine max Introduction Improving the yield of food crop production is one of the most important and urgent challenges for human being. This challenge requires the genetic diversity of crop for developing new crop varieties with both stress tolerance and high yield performance. However, the genetic diversity of crop has been decreased since the advent of modern agriculture. -

Taking Plant Pharmaceuticals from Research to Production
From Farm to Pharm: Taking plant pharmaceuticals from research to production Benjamin Doffek BSc, MSc (Hons) 0000-0001-7583-426X A thesis submitted for the degree of Doctor of Philosophy at The University of Queensland in 2020 Institute for Molecular Bioscience Abstract Abstract Affordable production of pharmaceuticals with high potency and low side effects is a major challenge of the 21st century. Peptides are an emerging class of therapeutics that have the potential to marry the specificity and efficacy of protein drugs with the stability and membrane permeability of small molecule drugs. Although many peptides are amenable to chemical synthesis, their cost of production is high, as is the generation of waste products. Peptide production in plants has the potential to be a scalable, cost effective, and a less environmentally taxing alternative. Cyclotides, first discovered in Oldenlandia affinis, are a unique class of backbone cyclic peptides containing three stabilising disulfide bridges that form a knot-like structure. Their stability, and for some variants, the ability to traverse cellular membranes make them ideal candidates for pharmaceutical and agricultural applications. Even though highly constrained sterically, cyclotides are amenable to engineering by replacing native sequences with bioactive epitopes. In this thesis, cyclotide production strategies in plant cell suspensions are examined with a special focus placed on O. affinis as an archetypical cyclotide producer. Additional species investigated are Clitoria ternatea, Hybanthus enneaspermus, Nicotiana benthamiana, and Petunia hybrida. Insights into how cyclotides and their biosynthetic processing machinery are regulated in suspension plant cells are reported and provide first steps towards an affordable and environmentally friendly peptide production system in plants. -

Parliamentary Bulletin
ParliamentaryRAJYA SABHA Bulletin PART-II No.59123-59126] MONDAY, SEPTEMBER 2, 2019 No.59123 Table Office Information/Documents to be furnished by Members and Leaders of Legislature parties in Rajya Sabha under the Anti-Defection Rules Under sub-rule (1) of Rule 3 of the Members of Rajya Sabha (Disqualification on Ground of Defection) Rules, 1985 made under paragraph 8 of the Tenth Schedule to the Constitution, Leader of each legislature party (other than a legislature party consisting of only one member) is required to furnish within thirty days from the date of commencement of these rules or, where such legislature party is formed after such date, within thirty days from the date of its formation, or, in either case, within such further period as the Chairman may for sufficient cause allow, the following to the Chairman, namely:— (a) a statement (in writing) containing the names of members of such legislature party together with other particulars regarding such members as in Form-I and the names and designations of the members of such party who have been authorized by it for communicating with the Chairman for purposes of these rules; (b) a copy of the rules and regulations (whether known as such or as constitution or by any other name) of the political party concerned; and (c) where such legislature party has any separate set of rules and regulations (whether known as such or as constitution or by any other name), also a copy of such rules and regulations. 2. Sub-rule (4) of rule 3 of the said rules provides that whenever any change takes place in the information furnished by the Leader of a legislature party, he/she is required to furnish in writing to the Chairman, information with respect to such change as soon as may be thereafter and in any case within thirty days from the date on which such change has taken place or within such further period as the Chairman may for sufficient cause allow. -
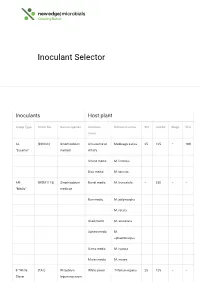
Inoculant Selector
Inoculant Selector Inoculants Host plant Group Type Strain No. Genus/species Common Botanical name Std Jumbo Mega Vial name AL (RRI128) Sinorhizobium All Lucerne or Medicago sativa 25 125 – 100 “Lucerne” meliloti Alfalfa Strand medic M. littoralis Disc medic M. tornata AM (WSM1115) Sinorhizobium Barrel medic M. truncatula – 250 – – “Medic” medicae Burr medic M. polymorpha M. rotata Snail medic M. scutellata Sphere medic M. sphaerocarpus Gama medic M. rugosa Murex medic M. murex B “White (TA1) Rhizobium White clover Trifolium repens 25 125 – – Clover leguminosarum bv. trifolii Red clover T. pratense Strawberry T. fragiferum clover Alsike clover T. hybridum Berseem, T. alexandrinum Egyptian clover Cluster, Ball T. glomeratum clover Suckling T. dubium clover C “Sub (WSM1325) Rhizobium Crimson Trifolium 50 250 – 200 Clover” leguminosarum clover incarnatum bv. trifolii Cupped clover T. cheleri Helmet clover T. clypeatum Rose clover T. hirtum Subterranean T. subterraneum clover var.subterraneum Subterranean T. subterraneum clover var.yanninicum Subterranean T. subterraneum clover var.brachy calycinum Arrowleaf T. vesiculosum 25 125 – 100 clover Balansa T. michelianum clover Bladder clover T. spumosum Gland clover T. glanduliferum Purple clover T. purpureum Persian clover T. resupinatum F “Faba & (WSM1455) Rhizobium Faba, Tick or Vicia faba – 500 1000 500 Pea” leguminosarum Broad bean bv. viciae Pea, Field pea Pisum sativum Common Vicia sativa – 500 1000 500 Vetch or Tare Bitter vetch V. ervilia Lathyrus Lathyruscicera Purple vetch V. benghalensis Woolly Pod V. villosa spp. vetch dasycarpa Lentil Lens culinaris – 250 500 250 G “Lupin” (WU425) Bradyrhizobium Narrow leaf Lupinus – 500 1000 500 sp. lupin angustifolius Mediterranean L. albus white lupin Yellow lupin L.