A Historical Study of Chemical Dosimetry (1902-1962)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Harry Moseley: Numbering the Elements
MYSTERY OF MATTER: SEARCH FOR THE ELEMENTS 5. Harry Moseley: Numbering the Elements Dmitri Mendeleev (identified on screen) works on the Periodic Table, writes down the atomic weights of the elements. NARR: Previously on The Mystery of Matter… HISTORIAN MICHAEL GORDIN VO He figures out something rather extraordinary about the elements. DMITRI MENDELEEV, partly in VO The eye is immediately struck by a pattern within the horizontal rows and the vertical columns. Mendeleev’s first table morphs into the familiar modern Periodic Table. AUTHOR ERIC SCERRI VO He found an absolutely fundamental principle of nature. Humphry Davy (identified on screen) performs an experiment with his voltaic pile. HISTORIAN DAVID KNIGHT VO Somehow the particles of matter have to be glued together to form molecules. What Davy has had is a big idea. Perhaps electricity could be this kind of glue. Marie Curie (identified on screen) sits down at the spectroscope and peers into the eyepiece. NARR: The spectroscope kicked off a whole new round in the discovery of elements. The spectra of four elements appear on screen, along with their names. PHYSICIST DAVID KAISER VO It’s almost like each element has its own bar code. Marie and Pierre enter their lab at night and see vials of radium glowing on the shelves. MARIE TO PIERRE Don’t light the lamps! Look! 1 MYSTERY OF MATTER: SEARCH FOR THE ELEMENTS PHYSICIST DAVID KAISER VO Radioactivity was a sign that the atom itself was unstable. It could break apart. Marie and Pierre look in wonder at their radiant element. NARR: Scientists now had a pressing new question to answer: What’s inside the atom? Fade to black ANNOUNCER: Major funding for The Mystery of Matter: Search for the Elements was provided by the National Science Foundation, where discoveries begin. -

Radiation and Radioactivity Quantified? Do You Think of These “People” When I Say RADIATION? Do You Think of These Things As Well?
Welcome To RadTown USA •Click to Explore RadTown USA • Click on any location in RadTown USA and find out about radiation sources or uses at that location. The Alpha, Beta, Gammas of Nuclear Education March 2nd, 2014 Fundamentals of Ionizing Radiation Debra N Thrall, PhD Executive Director Albert I Pierce Foundation Radiation Fundamentals What is radiation? Where does it come from? How does it interact with matter? What is radioactivity? What are fission and fusion? How are radiation and radioactivity quantified? Do you think of these “people” when I say RADIATION? Do you think of these things as well? • Food • Space • Utilities • Consumer Products • Medicine Brief History of the Atom • 500 BC Democritus Atom • Long time (Romans Dark Ages) • 1808 AD Dalton Plum Pudding • 1911 Rutherford Nucleus • 1913 Bohr Orbits • 1920’s Many People Quantum Mechanics Rutherford’s Gold Foil Experiment The Design 1. Bombard positively charged alpha particles into thin gold foil. 2. Use fluorescent screen to detect particles as they exit the gold foil. 3. Use angle of deflection to determine interior of the atom. So, What is an Atom? • Atoms are made up of protons, neutrons & electrons • Protons: + charge p+ • Neutrons: no charge n0 • Electrons: - charge e- • Atoms want to have a stable energy level • This translates to having no net charge • # protons = # electrons Mass of an Atom • Masses • Proton: 1.000000 amu • Neutron: 1.000000 amu • Electron: 0.000549 amu (Translates to 1.2 lbs/1 ton ~ a kitten on an elephant!) • The mass of an atom is approximately -

The International Commission on Radiological Protection: Historical Overview
Topical report The International Commission on Radiological Protection: Historical overview The ICRP is revising its basic recommendations by Dr H. Smith Within a few weeks of Roentgen's discovery of gamma rays; 1.5 roentgen per working week for radia- X-rays, the potential of the technique for diagnosing tion, affecting only superficial tissues; and 0.03 roentgen fractures became apparent, but acute adverse effects per working week for neutrons. (such as hair loss, erythema, and dermatitis) made hospital personnel aware of the need to avoid over- Recommendations in the 1950s exposure. Similar undesirable acute effects were By then, it was accepted that the roentgen was reported shortly after the discovery of radium and its inappropriate as a measure of exposure. In 1953, the medical applications. Notwithstanding these observa- ICRU recommended that limits of exposure should be tions, protection of staff exposed to X-rays and gamma based on consideration of the energy absorbed in tissues rays from radium was poorly co-ordinated. and introduced the rad (radiation absorbed dose) as a The British X-ray and Radium Protection Committee unit of absorbed dose (that is, energy imparted by radia- and the American Roentgen Ray Society proposed tion to a unit mass of tissue). In 1954, the ICRP general radiation protection recommendations in the introduced the rem (roentgen equivalent man) as a unit early 1920s. In 1925, at the First International Congress of absorbed dose weighted for the way different types of of Radiology, the need for quantifying exposure was radiation distribute energy in tissue (called the dose recognized. As a result, in 1928 the roentgen was equivalent in 1966). -

MIRD Pamphlet No. 22 - Radiobiology and Dosimetry of Alpha- Particle Emitters for Targeted Radionuclide Therapy
Alpha-Particle Emitter Dosimetry MIRD Pamphlet No. 22 - Radiobiology and Dosimetry of Alpha- Particle Emitters for Targeted Radionuclide Therapy George Sgouros1, John C. Roeske2, Michael R. McDevitt3, Stig Palm4, Barry J. Allen5, Darrell R. Fisher6, A. Bertrand Brill7, Hong Song1, Roger W. Howell8, Gamal Akabani9 1Radiology and Radiological Science, Johns Hopkins University, Baltimore MD 2Radiation Oncology, Loyola University Medical Center, Maywood IL 3Medicine and Radiology, Memorial Sloan-Kettering Cancer Center, New York NY 4International Atomic Energy Agency, Vienna, Austria 5Centre for Experimental Radiation Oncology, St. George Cancer Centre, Kagarah, Australia 6Radioisotopes Program, Pacific Northwest National Laboratory, Richland WA 7Department of Radiology, Vanderbilt University, Nashville TN 8Division of Radiation Research, Department of Radiology, New Jersey Medical School, University of Medicine and Dentistry of New Jersey, Newark NJ 9Food and Drug Administration, Rockville MD In collaboration with the SNM MIRD Committee: Wesley E. Bolch, A Bertrand Brill, Darrell R. Fisher, Roger W. Howell, Ruby F. Meredith, George Sgouros (Chairman), Barry W. Wessels, Pat B. Zanzonico Correspondence and reprint requests to: George Sgouros, Ph.D. Department of Radiology and Radiological Science CRB II 4M61 / 1550 Orleans St Johns Hopkins University, School of Medicine Baltimore MD 21231 410 614 0116 (voice); 413 487-3753 (FAX) [email protected] (e-mail) - 1 - Alpha-Particle Emitter Dosimetry INDEX A B S T R A C T......................................................................................................................... -
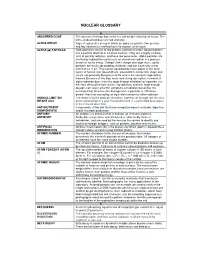
Nuclear Glossary
NUCLEAR GLOSSARY A ABSORBED DOSE The amount of energy deposited in a unit weight of biological tissue. The units of absorbed dose are rad and gray. ALPHA DECAY Type of radioactive decay in which an alpha ( α) particle (two protons and two neutrons) is emitted from the nucleus of an atom. ALPHA (ααα) PARTICLE. Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus. They are a highly ionizing form of particle radiation, and have low penetration. Alpha particles are emitted by radioactive nuclei such as uranium or radium in a process known as alpha decay. Owing to their charge and large mass, alpha particles are easily absorbed by materials and can travel only a few centimetres in air. They can be absorbed by tissue paper or the outer layers of human skin (about 40 µm, equivalent to a few cells deep) and so are not generally dangerous to life unless the source is ingested or inhaled. Because of this high mass and strong absorption, however, if alpha radiation does enter the body through inhalation or ingestion, it is the most destructive form of ionizing radiation, and with large enough dosage, can cause all of the symptoms of radiation poisoning. It is estimated that chromosome damage from α particles is 100 times greater than that caused by an equivalent amount of other radiation. ANNUAL LIMIT ON The intake in to the body by inhalation, ingestion or through the skin of a INTAKE (ALI) given radionuclide in a year that would result in a committed dose equal to the relevant dose limit . -

Chapter 2 Radiation Safety Manual Revision 1 Principles of Radiation Safety 6/1/2018
The University of Georgia Chapter 2 Radiation Safety Manual Revision 1 Principles of Radiation Safety 6/1/2018 CHAPTER 2 PRINCIPLES OF RADIATION SAFETY 1.0 UNITS OF RADIATION DOSE 1.1 Historical Units In 1896, x-rays were discovered by Wilhelm Roentgen. The Roentgen is unit of measurement for the exposure of x-rays and gamma rays. It is defined as the electric charge freed by such radiation in a specified volume of air divided by the mass of that air. Radioactivity was discovered by Henri Becquerel. Radioactivity refers to the amount of radiation released when an element spontaneously emits energy as a result of the decay of its unstable atoms. The Becquerel is a unit used to measure radioactivity, and stands for 1 disintegration per second. The Curie is a unit of radioactivity equal to 3.7 X 1010 disintegrations per second and was first used, but being such a large unit was replaced by the Becquerel. The Curie is named for Pierre Curie, Marie Curie’s husband and co- discoverer of radium. After his accidental death, she continued their work. For some time, no one realized that radiation could cause harmful effects. It was recognized very soon that x-rays could be used in medical diagnosis, and early radiologists received large doses of radiation. Many of these radiologists later suffered severe injuries due to overexposure (radiation effects may appear years after exposure). The first unit of dose was the erythema dose. This was the amount of x-radiation which would barely cause reddening of the skin. It was not a very satisfactory unit of dose, but indicates the early recognition by some scientists that radiation exposure can be harmful and should be limited. -

Radiation Glossary
Radiation Glossary Activity The rate of disintegration (transformation) or decay of radioactive material. The units of activity are Curie (Ci) and the Becquerel (Bq). Agreement State Any state with which the U.S. Nuclear Regulatory Commission has entered into an effective agreement under subsection 274b. of the Atomic Energy Act of 1954, as amended. Under the agreement, the state regulates the use of by-product, source, and small quantities of special nuclear material within said state. Airborne Radioactive Material Radioactive material dispersed in the air in the form of dusts, fumes, particulates, mists, vapors, or gases. ALARA Acronym for "As Low As Reasonably Achievable". Making every reasonable effort to maintain exposures to ionizing radiation as far below the dose limits as practical, consistent with the purpose for which the licensed activity is undertaken. It takes into account the state of technology, the economics of improvements in relation to state of technology, the economics of improvements in relation to benefits to the public health and safety, societal and socioeconomic considerations, and in relation to utilization of radioactive materials and licensed materials in the public interest. Alpha Particle A positively charged particle ejected spontaneously from the nuclei of some radioactive elements. It is identical to a helium nucleus, with a mass number of 4 and a charge of +2. Annual Limit on Intake (ALI) Annual intake of a given radionuclide by "Reference Man" which would result in either a committed effective dose equivalent of 5 rems or a committed dose equivalent of 50 rems to an organ or tissue. Attenuation The process by which radiation is reduced in intensity when passing through some material. -

External and Internal Dosimetry
Chapter 7 External and Internal Dosimetry H-117 – Introductory Health Physics Slide 1 Objectives ¾ Discuss factors influencing external and internal doses ¾ Define terms used in external dosimetry ¾ Discuss external dosimeters such as TLDs, film badges, OSL dosimeters, pocket chambers, and electronic dosimetry H-117 – Introductory Health Physics Slide 2 Objectives ¾ Define terms used in internal dosimetry ¾ Discuss dose units and limits ¾ Define the ALI, DAC and DAC-hr ¾ Discuss radiation signs and postings H-117 – Introductory Health Physics Slide 3 Objectives ¾ Discuss types of bioassays ¾ Describe internal dose measuring equipment and facilities ¾ Discuss principles of internal dose calculation and work sample problems H-117 – Introductory Health Physics Slide 4 External Dosimetry H-117 – Introductory Health Physics Slide 5 External Dosimetry Gamma, beta or neutron radiation emitted by radioactive material outside the body irradiates the skin, lens of the eye, extremities & the whole body (i.e. internal organs) H-117 – Introductory Health Physics Slide 6 External Dosimetry (cont.) ¾ Alpha particles cannot penetrate the dead layer of skin (0.007 cm) ¾ Beta particles are primarily a skin hazard. However, energetic betas can penetrate the lens of an eye (0.3 cm) and deeper tissue (1 cm) ¾ Beta sources can produce more penetrating radiation through bremsstrahlung ¾ Primary sources of external exposure are photons and neutrons ¾ External dose must be measured by means of appropriate dosimeters H-117 – Introductory Health Physics Slide 7 -

Evaluation of Fernald
Draft ADVISORY BOARD ON RADIATION AND WORKER HEALTH National Institute for Occupational Safety and Health Review of the General Steel Industries Special Exposure Cohort (SEC) Petition-00105 and the NIOSH SEC Petition Evaluation Report Contract No. 200-2009-28555 SCA-SEC-TASK5-0005 Prepared by S. Cohen & Associates 1608 Spring Hill Road, Suite 400 Vienna, Virginia 22182 July 2009 [Redacted version – October 2, 2009] Disclaimer This document is made available in accordance with the unanimous desire of the Advisory Board on Radiation and Worker Health (ABRWH) to maintain all possible openness in its deliberations. However, the ABRWH and its contractor, SC&A, caution the reader that at the time of its release, this report is pre-decisional and has not been reviewed by the Board for factual accuracy or applicability within the requirements of 42 CFR 82. This implies that once reviewed by the ABRWH, the Board’s position may differ from the report’s conclusions. Thus, the reader should be cautioned that this report is for information only and that premature interpretations regarding its conclusions are unwarranted. Effective Date: Revision No.: Document No.: Page No.: July 24, 2009 0 SCA-SEC-TASK5-0005 Page 2 of 50 S. COHEN & ASSOCIATES: Document No. SCA-SEC-TASK5-0005 Technical Support for the Advisory Board on Effective Date: Radiation & Worker Health Review of July 24, 2009 – Draft NIOSH Dose Reconstruction Program Redacted version – October 2, 2009 Revision No. 0 Review of the General Steel Industries Special Exposure Cohort (SEC) Petition-00105 -

A Standard Complex-Wide Methodology for Overestimating External Doses Effective Date: 06/05/2006 Measured with Film Badge Dosimeters Type of Document: OTIB
ORAU TEAM Dose Reconstruction Project for NIOSH Oak Ridge Associated Universities I Dade Moeller & Associates I MJW Corporation Page 1 of 19 Document Title: Document Number: ORAUT-OTIB-0010 Revision: 01 A Standard Complex-Wide Methodology for Overestimating External Doses Effective Date: 06/05/2006 Measured with Film Badge Dosimeters Type of Document: OTIB Supersedes: Revision 00 Subject Experts: Donald N. Stewart and Jack J. Fix Document Owner Approval: Signature on File Approval Date: 05/17/2006 James P. Griffin, Deputy Project Director Concurrence: Signature on File Concurrence Date: 05/22/2006 Kate Kimpan, Project Director Approval: Signature on File Approval Date: 06/05/2006 James W. Neton, Associate Director for Science New Total Rewrite Revision Page Change FOR DOCUMENTS MARKED AS A TOTAL REWRITE, REVISION, OR PAGE CHANGE, REPLACE THE PRIOR REVISION AND DISCARD / DESTROY ALL COPIES OF THE PRIOR REVISION. Document No. ORAUT-OTIB-0010 Revision No. 01 Effective Date: 06/05/2006 Page 2 of 19 PUBLICATION RECORD EFFECTIVE REVISION DATE NUMBER DESCRIPTION 01/12/2004 00 New Technical Information Bulletin for a Standard Complex-Wide Correction Factor for Overestimating External Doses Measured with Film Badge Dosimeters. First approved issue. Initiated by Donald N. Stewart. 06/05/2006 01 Constitutes rewrite of document as a result of biennial review. Incorporates resolution of S. Cohen & Associates final draft comments contained in “The Review of NIOSH/ORAUT Procedures and Methods used for Dose Reconstruction” (ref: letter from John Mauro to David Staudt, dated January 17, 2005). Approved issue of Revision 01. This revision results in no change to the assigned dose and no PER is required. -

11. Dosimetry Fundamentals
Outline • Introduction Dosimetry Fundamentals • Dosimeter model • Interpretation of dosimeter measurements Chapter 11 – Photons and neutrons – Charged particles • General characteristics of dosimeters F.A. Attix, Introduction to Radiological Physics and Radiation Dosimetry • Summary Introduction Dosimeter • Radiation dosimetry deals with the determination • A dosimeter can be generally defined as (i.e., by measurement or calculation) of the any device that is capable of providing a absorbed dose or dose rate resulting from the interaction of ionizing radiation with matter reading R that is a measure of the absorbed • Other radiologically relevant quantities are dose Dg deposited in its sensitive volume V exposure, kerma, fluence, dose equivalent, energy by ionizing radiation imparted, etc. can be determined • If the dose is not homogeneous • Measuring one quantity (usually the absorbed dose) another one can be derived through throughout the sensitive calculations based on the defined relationships volume, then R is a measure of mean value Dg Dosimeter Simple dosimeter model • Ordinarily one is not interested in measuring • A dosimeter can generally be considered as the absorbed dose in a dosimeter’s sensitive consisting of a sensitive volume V filled with a volume itself, but rather as a means of medium g, surrounded by a wall (or envelope, determining the dose (or a related quantity) for container, etc.) of another medium w having a another medium in which direct measurements thickness t 0 are not feasible • A simple dosimeter can be -

Q: What's the Difference Between Roentgen, Rad and Rem Radiation Measurements?
www.JICReadiness.com Q: What's the Difference Between Roentgen, Rad and Rem Radiation Measurements? A: Since nuclear radiation affects people, we must be able to measure its presence. We also need to relate the amount of radiation received by the body to its physiological effects. Two terms used to relate the amount of radiation received by the body are exposure and dose. When you are exposed to radiation, your body absorbs a dose of radiation. As in most measurement quantities, certain units are used to properly express the measurement. For radiation measurements they are... Roentgen: The roentgen measures the energy produced by gamma radiation in a cubic centimeter of air. It is usually abbreviated with the capital letter "R". A milliroentgen, or "mR", is equal to one one-thousandth of a roentgen. An exposure of 50 roentgens would be written "50 R". Rad: Or, Radiation Absorbed Dose recognizes that different materials that receive the same exposure may not absorb the same amount of energy. A rad measures the amount of radiation energy transferred to some mass of material, typically humans. One roentgen of gamma radiation exposure results in about one rad of absorbed dose. Rem: Or, Roentgen Equivalent Man is a unit that relates the dose of any radiation to the biological effect of that dose. To relate the absorbed dose of specific types of radiation to their biological effect, a "quality factor" must be multiplied by the dose in rad, which then shows the dose in rems. For gamma rays and beta particles, 1 rad of exposure results in 1 rem of dose.