The Pipevine Swallowtail: Life Cycle and Ecology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Vascular Plants of Massachusetts
The Vascular Plants of Massachusetts: The Vascular Plants of Massachusetts: A County Checklist • First Revision Melissa Dow Cullina, Bryan Connolly, Bruce Sorrie and Paul Somers Somers Bruce Sorrie and Paul Connolly, Bryan Cullina, Melissa Dow Revision • First A County Checklist Plants of Massachusetts: Vascular The A County Checklist First Revision Melissa Dow Cullina, Bryan Connolly, Bruce Sorrie and Paul Somers Massachusetts Natural Heritage & Endangered Species Program Massachusetts Division of Fisheries and Wildlife Natural Heritage & Endangered Species Program The Natural Heritage & Endangered Species Program (NHESP), part of the Massachusetts Division of Fisheries and Wildlife, is one of the programs forming the Natural Heritage network. NHESP is responsible for the conservation and protection of hundreds of species that are not hunted, fished, trapped, or commercially harvested in the state. The Program's highest priority is protecting the 176 species of vertebrate and invertebrate animals and 259 species of native plants that are officially listed as Endangered, Threatened or of Special Concern in Massachusetts. Endangered species conservation in Massachusetts depends on you! A major source of funding for the protection of rare and endangered species comes from voluntary donations on state income tax forms. Contributions go to the Natural Heritage & Endangered Species Fund, which provides a portion of the operating budget for the Natural Heritage & Endangered Species Program. NHESP protects rare species through biological inventory, -

Volume 12 - Number 1 March 2005
Utah Lepidopterist Bulletin of the Utah Lepidopterists' Society Volume 12 - Number 1 March 2005 Extreme Southwest Utah Could See Iridescent Greenish-blue Flashes A Little Bit More Frequently by Col. Clyde F. Gillette Battus philenor (blue pipevine swallowtail) flies in the southern two- thirds of Arizona; in the Grand Canyon (especially at such places as Phantom Ranch 8/25 and Indian Gardens 12/38) and at its rims [(N) 23/75 and (S) 21/69]; in the low valleys of Clark Co., Nevada; and infrequently along the Meadow Valley Wash 7/23 which parallels the Utah/Nevada border in Lincoln Co., Nevada. Since this beautiful butterfly occasionally flies to the west, southwest, and south of Utah's southwest corner, one might expect it to turn up now and then in Utah's Mojave Desert physiographic subsection of the Basin and Range province on the lower southwest slopes of the Beaver Dam Mountains, Battus philenor Blue Pipevine Swallowtail Photo courtesy of Randy L. Emmitt www.rlephoto.com or sporadically fly up the "Dixie Corridor" along the lower Virgin River Valley. Even though both of these Lower Sonoran life zone areas reasons why philenor is not a habitual pipevine species.) Arizona's of Utah offer potentially suitable, resident of Utah's Dixie. But I think interesting plant is Aristolochia "nearby" living conditions for Bat. there is basically only one, and that is watsonii (indianroot pipevine), which phi. philenor, such movements have a complete lack of its larval has alternate leaves shaped like a not often taken place. Or, more foodplants in the region. -

Butterflies of the Finger Lakes National Forest
The Finger Lakes National Butterflies of "... the first precaution Forest comprises 16,000 acres of the of intelligent tinkering federally owned land between Cayuga and Seneca Lakes in Finger Lakes is to keep every cog and New York State. The Forest is wheel ... " managed for multiple uses to ~~Mut provide a variety of goods and ~Aldo Leopold services to a diverse public. Different programs include a This list compiled through the work of livestock grazing program, a Charles R. Smith, Ph.D. Department of Natural Resources small scale timber management Cornell University program, diverse habitats for And Donald Bright-Smith, Ph.D. wildlife and fish, and Department of Pathobiology recreational opportunities for TexasA&M multiple user groups. And made possible through the generosity New York State Chapter of the For more information about the Finger Lakes Wild Turkey Federation National Forest contact: Hector Ranger District 5218 State Route 414 United States Forest Eastern ~~1~ .. ~ Department of Service Region New York State Chapter Hector, NY 14841 Agriculture NWTF (607) 546-4470 Family Papilionidae: Swallowtails Family Hesperiidae: Skippers 0 Papilio polyxenes, Black Swallowtail 0 Epargyreus clarus, Silver-spotted 0 Pap ilia cresphontes, Giant Swallowtail Skipper 0 Papilio glaucus, Eastern Tiger Family Nymphalidae: Brushfoots 0 Erynnis ice/us, Dreamy Duskywing Swallowtail 0 Speyeria cybele, Great Spangled Fritillary D Erynnisjuvenalis, Juvenal's Duskywing 0 Pap ilia troilus, Spicebush Swallowtail 0 Boloria selene, Silver-bordered Fritillary -

Aristolochic Acids Affect the Feeding Behaviour and Development of Battus Polydamas Archidamas Larvae (Lepidoptera: Papilionidae: Troidini)
Eur. J. Entomol. 106: 357–361, 2009 http://www.eje.cz/scripts/viewabstract.php?abstract=1462 ISSN 1210-5759 (print), 1802-8829 (online) Aristolochic acids affect the feeding behaviour and development of Battus polydamas archidamas larvae (Lepidoptera: Papilionidae: Troidini) CARLOS F. PINTO1, ALEJANDRA J. TRONCOSO1, ALEJANDRO URZÚA2 and HERMANN M. NIEMEYER1* 1 Departamento de Ciencias Ecológicas, Facultad de Ciencias, Universidad de Chile, Casilla 653, Santiago, Chile 2 Facultad de Química y Biología, Universidad de Santiago de Chile, Casilla 40, Santiago-33, Chile Key words. Lepidoptera, Papilionidae, Battus polydamas archidamas, Aristolochia chilensis, aristolochic acid content, foraging substrate, larval development Abstract. The feeding behaviour of specialist butterflies may be affected by the mechanical and chemical characteristics of the tis- sues of their host-plants. Larvae of the butterfly, Battus polydamas archidamas feed only on Aristolochia chilensis, which contains aristolochic acids. We studied the oviposition pattern of adults and the foraging of larvae of B. polydamas archidamas over time in relation to variations in hardness of the substrate and concentration of aristolochic acids in different plant tissues. We further tested the effect of two artificial diets containing different concentrations of aristolochic acids on larval performance. B. polydamas archi- damas oviposited mostly on young leaves and the larvae fed on this tissue until the second instar. Third instar larvae fed also on mature leaves and fourth and higher instars fed also on stems. Young leaves are softer and contain higher concentrations of aris- tolochic acids than mature leaves, and stems are both harder and contain a high concentration of aristolochic acids. Larvae reared on artificial diets containing a high concentration of aristolochic acids suffered less mortality and were heavier than those reared on a diet with a lower concentration of aristolochic acids, which suggests they are phagostimulatory. -

Butterfly Gardening Tips & Tricks Gardening for Butterflies Is Fun, Beautiful, and Good for the Environment
Butterfly Gardening Tips & Tricks Gardening for butterflies is fun, beautiful, and good for the environment. It is also simple and can be done in almost any location. The key guidelines are listed below: NO PESTICIDES! Caterpillars are highly susceptible to almost all pesticides so keep them away from your yard if you want butterflies to thrive. Select the right plants. You will need to provide nectar sources for adults and host plants for caterpillars. See the lists below for inspiration. Keep to native varieties as much as possible. Plants come in lots and lots of varieties and cultivars. When selecting plants, especially host plants, try to find native species as close to the natural or wild variety as possible. Provide shelter. Caterpillars need shelter from the sun and shelter from cold nights. Adults need places to roost during the night. And protected areas are needed for the chrysalis to safely undergo its transformation. The best way to provide shelter is with large clumps of tall grasses (native or ornamental) and medium to large evergreen trees and/or shrubs. Nectar Sources Top Ten Nectar Sources: Asclepias spp. (milkweed) Aster spp. Buddleia spp. (butterfly bush) Coreopsis spp. Echinacea spp. (coneflower) Eupatorium spp. (joe-pye weed) Lantana spp. Liatris spp. Pentas spp. Rudbeckia spp. (black-eyed susan) Others: Agastache spp. (hyssop), Apocynum spp. (dogbane), Ceanothus americanus (New Jersey tea), Cephalanthus occidentalis (button bush), Clethra alnifolia, Cuphea spp. (heather), Malus spp. (apple), Mentha spp. (mint), Phlox spp., Pycanthemum incanum (mountain mint), Salivs spp. (sage), Sedum spectabile (stonecrop), Stokesia laevis (cornflower), Taraxacum officinale (dandelion), Triofolium spp. -
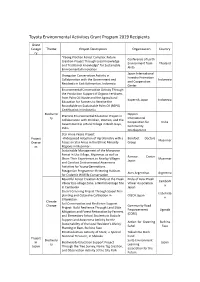
List of Previous Grant Projects
Toyota Environmental Activities Grant Program 2019 Recipients Grant Catego Theme Project Description Organization Country ry "Kaeng Krachan Forest Complex: Future Conference of Earth Creation Project Through Local Knowledge Environment from Thailand and Traditional Knowledge" for Sustainable Akita Environmental Innovation Japan International Orangutan Conservation Activity in Forestry Promotion Collaboration with the Government and Indonesia and Cooperation Residents in East Kalimantan, Indonesia Center Environmental Conservation Activity Through the Production Support of Organic Fertilizers from Palm Oil Waste and the Agricultural Kopernik Japan Indonesia Education for Farmers to Receive the Roundtable on Sustainable Palm Oil (RSPO) Certification in Indonesia Biodiversi Nippon Practical Environmental Education Project in ty International Collaboration with Children, Women, and the Cooperation for India Government in a Rural Village in Bodh Gaya, Community India Development Star Anise Peace Project Project -Widespread Adoption of Agroforestry with a Barefoot Doctors Myanmar Overse Focus on Star Anise in the Ethnic Minority Group as Regions in Myanmar- Sustainable Management of the Mangrove Forest in Uto Village, Myanmar, as well as Ramsar Center Share Their Experiences to Nearby Villages Myanmar Japan and Conduct Environmental Awareness Activities for Young Generations Patagonian Programme: Restoring Habitats Aves Argentinas Argentina for Endemic Wildlife Conservation Beautiful Forest Creation Activity at the Preah Pride of Asia: Preah -

Aristolochia Serpentaria L
New England Plant Conservation Program Aristolochia serpentaria L. Virginia Snakeroot Conservation and Research Plan for New England Prepared by: Dorothy J. Allard, Ph.D. Analytical Resources, LLC P.O. Box 279 East Montpelier, VT 05651 For: New England Wild Flower Society 180 Hemenway Road Framingham, MA 01701 508/877-7630 e-mail: [email protected] • website: www.newfs.org Approved, Regional Advisory Council, 2002 SUMMARY Aristolochia serpentaria L., commonly known as Virginia Snakeroot, is a perennial herb in the Aristolochiaceae. Several varieties have been described in the past, but they are no longer recognized in most taxonomic manuals. Aristolochia serpentaria occurs in 26 eastern states and is common in the southern and central part of its range, while becoming rare along its northern periphery. It is listed as a Division 2 species in Flora Conservanda (Brumback and Mehrhoff et al. 1996). Connecticut is the only New England state in which it is found, where it occurs at 12 known sites in ten towns. It grows in a variety of upland forest communities, but is more likely to be found in dry, somewhat rich, rocky, deciduous or mixed deciduous-coniferous woods in Connecticut. Farther south, it also has broad environmental requirements, occupying many upland soil and forest types. Population trends for the species in Connecticut are unclear. One new population was discovered in 2001. Monitoring in 2001 of eight extant sites found that two populations had decreased, three had increased, and one had stayed the same. Several known populations are threatened by habitat modification, invasive species, or site fragility. Two extant populations have not been surveyed for several years. -

Arthropods of Elm Fork Preserve
Arthropods of Elm Fork Preserve Arthropods are characterized by having jointed limbs and exoskeletons. They include a diverse assortment of creatures: Insects, spiders, crustaceans (crayfish, crabs, pill bugs), centipedes and millipedes among others. Column Headings Scientific Name: The phenomenal diversity of arthropods, creates numerous difficulties in the determination of species. Positive identification is often achieved only by specialists using obscure monographs to ‘key out’ a species by examining microscopic differences in anatomy. For our purposes in this survey of the fauna, classification at a lower level of resolution still yields valuable information. For instance, knowing that ant lions belong to the Family, Myrmeleontidae, allows us to quickly look them up on the Internet and be confident we are not being fooled by a common name that may also apply to some other, unrelated something. With the Family name firmly in hand, we may explore the natural history of ant lions without needing to know exactly which species we are viewing. In some instances identification is only readily available at an even higher ranking such as Class. Millipedes are in the Class Diplopoda. There are many Orders (O) of millipedes and they are not easily differentiated so this entry is best left at the rank of Class. A great deal of taxonomic reorganization has been occurring lately with advances in DNA analysis pointing out underlying connections and differences that were previously unrealized. For this reason, all other rankings aside from Family, Genus and Species have been omitted from the interior of the tables since many of these ranks are in a state of flux. -

Sequestration of Aristolochic Acids from Meridic Diets by Larvae of Battus Polydamas Archidamas (Papilionidae: Troidini)
Eur. J. Entomol. 108: 41–45, 2011 http://www.eje.cz/scripts/viewabstract.php?abstract=1585 ISSN 1210-5759 (print), 1802-8829 (online) Sequestration of aristolochic acids from meridic diets by larvae of Battus polydamas archidamas (Papilionidae: Troidini) CARLOS F. PINTO1, ALEJANDRO URZÚA2 and HERMANN M. NIEMEYER1* 1Laboratorio de Química Ecológica, Universidad de Chile, Casilla 653, Santiago, Chile 2Laboratorio de Química Ecológica, Universidad de Santiago de Chile, Casilla 40, C-33 Santiago, Chile Key words. Lepidoptera, Papilionidae, Battus polydamas archidamas, Aristolochia chilensis, aristolochic acids, sequestration of toxins, uptake of toxins Abstract. Larvae of the butterfly, Battus polydamas archidamas (Papilionidae: Troidini) feed exclusively on aristolochic acid (AAs)-containing Aristolochia species (Aristolochiaceae). The distribution of sequestrated AAs in the tissues (body, integument and osmeterial secretions) of B. polydamas archidamas larvae during their development, when fed on a meridic diet containing either a higher or lower concentration of AAs (AAI and AAII) than occurs naturally in the aerial tissues of their host plant, was determined. Accumulation of AAs in the body and integument was proportional to the weight of larvae and greater in the larvae that fed on the diet containing the higher concentration of AAs. Phenolic AAs (AAIa and AAIVa) not present in the diets were found in all larval tissues examined. Integument and body extracts had a higher AAI/AAII ratio than in the original diet and also a relatively high AAIa/AAIVa ratio, suggesting a preferred AAII to AAIa transformation in those larval tissues. In the osmeterial secretion, the value of the AAI/AAII ratio was similar to that in the diets and the AAIa/AAIVa ratio close to 1, which suggests that hydroxylation of AAI to AAIVa and of AAII to AAIa occur to similar extents. -

Reconstructing the Basal Angiosperm Phylogeny: Evaluating Information Content of Mitochondrial Genes
55 (4) • November 2006: 837–856 Qiu & al. • Basal angiosperm phylogeny Reconstructing the basal angiosperm phylogeny: evaluating information content of mitochondrial genes Yin-Long Qiu1, Libo Li, Tory A. Hendry, Ruiqi Li, David W. Taylor, Michael J. Issa, Alexander J. Ronen, Mona L. Vekaria & Adam M. White 1Department of Ecology & Evolutionary Biology, The University Herbarium, University of Michigan, Ann Arbor, Michigan 48109-1048, U.S.A. [email protected] (author for correspondence). Three mitochondrial (atp1, matR, nad5), four chloroplast (atpB, matK, rbcL, rpoC2), and one nuclear (18S) genes from 162 seed plants, representing all major lineages of gymnosperms and angiosperms, were analyzed together in a supermatrix or in various partitions using likelihood and parsimony methods. The results show that Amborella + Nymphaeales together constitute the first diverging lineage of angiosperms, and that the topology of Amborella alone being sister to all other angiosperms likely represents a local long branch attrac- tion artifact. The monophyly of magnoliids, as well as sister relationships between Magnoliales and Laurales, and between Canellales and Piperales, are all strongly supported. The sister relationship to eudicots of Ceratophyllum is not strongly supported by this study; instead a placement of the genus with Chloranthaceae receives moderate support in the mitochondrial gene analyses. Relationships among magnoliids, monocots, and eudicots remain unresolved. Direct comparisons of analytic results from several data partitions with or without RNA editing sites show that in multigene analyses, RNA editing has no effect on well supported rela- tionships, but minor effect on weakly supported ones. Finally, comparisons of results from separate analyses of mitochondrial and chloroplast genes demonstrate that mitochondrial genes, with overall slower rates of sub- stitution than chloroplast genes, are informative phylogenetic markers, and are particularly suitable for resolv- ing deep relationships. -

JUDD W.S. Et. Al. (1999) Plant Systematics
CHAPTER8 Phylogenetic Relationships of Angiosperms he angiosperms (or flowering plants) are the dominant group of land Tplants. The monophyly of this group is strongly supported, as dis- cussed in the previous chapter, and these plants are possibly sister (among extant seed plants) to the gnetopsids (Chase et al. 1993; Crane 1985; Donoghue and Doyle 1989; Doyle 1996; Doyle et al. 1994). The angio- sperms have a long fossil record, going back to the upper Jurassic and increasing in abundance as one moves through the Cretaceous (Beck 1973; Sun et al. 1998). The group probably originated during the Jurassic, more than 140 million years ago. Cladistic analyses based on morphology, rRNA, rbcL, and atpB sequences do not support the traditional division of angiosperms into monocots (plants with a single cotyledon, radicle aborting early in growth with the root system adventitious, stems with scattered vascular bundles and usually lacking secondary growth, leaves with parallel venation, flow- ers 3-merous, and pollen grains usually monosulcate) and dicots (plants with two cotyledons, radicle not aborting and giving rise to mature root system, stems with vascular bundles in a ring and often showing sec- ondary growth, leaves with a network of veins forming a pinnate to palmate pattern, flowers 4- or 5-merous, and pollen grains predominantly tricolpate or modifications thereof) (Chase et al. 1993; Doyle 1996; Doyle et al. 1994; Donoghue and Doyle 1989). In all published cladistic analyses the “dicots” form a paraphyletic complex, and features such as two cotyle- dons, a persistent radicle, stems with vascular bundles in a ring, secondary growth, and leaves with net venation are plesiomorphic within angio- sperms; that is, these features evolved earlier in the phylogenetic history of tracheophytes. -

Natural History of Fiji's Endemic Swallowtail Butterfly, Papilio Schmeltzi
32 TROP. LEPID. RES., 23(1): 32-38, 2013 CHANDRA ET AL.: Life history of Papilio schmeltzi NATURAL HISTORY OF FIJI’S ENDEMIC SWALLOWTAIL BUTTERFLY, PAPILIO SCHMELTZI (HERRICH-SCHAEFFER) Visheshni Chandra1, Uma R. Khurma1 and Takashi A. Inoue2 1School of Biological and Chemical Sciences, Faculty of Science, Technology and Environment, The University of the South Pacific, Private Bag, Suva, Fiji. Correspondance: [email protected]; 2Japanese National Institute of Agrobiological Sciences, Ôwashi 1-2, Tsukuba, Ibaraki, 305-8634, Japan Abstract - The wild population of Papilio schmeltzi (Herrich-Schaeffer) in the Fiji Islands is very small. Successful rearing methods should be established prior to any attempts to increase numbers of the natural population. Therefore, we studied the biology of this species. Papilio schmeltzi was reared on Micromelum minutum. Three generations were reared during the period from mid April 2008 to end of November 2008, and hence we estimate that in nature P. schmeltzi may have up to eight generations in a single year. Key words: Papilio schmeltzi, Micromelum minutum, life cycle, larval host plant, developmental duration, morphological characters, captive breeding INTRODUCTION MATERIALS AND METHODS Most of the Asia-Pacific swallowtail butterflies P. schmeltzi was reared in a screened enclosure from mid (Lepidopera: Papilionidae) belonging to the genus Papilio are April 2008 to end of November 2008. The enclosure was widely distributed in the tropics (e.g. Asia, Papua New Guinea, designed to provide conditions as close to its natural habitat as Australia, New Caledonia, Vanuatu, Solomon Islands, Fiji and possible and was located in an open area at the University of Samoa).