Bioactive Components in Fish Venoms
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Very Long Term Tag Recovery of a California Scorpionfish (Scorpaena Guttata)
California Fish and Game 105(1):8-9; 2019 A very long term tag recovery of a California Scorpionfish (Scorpaena guttata) EDGAR W. ROBERTS III* AND DOYLE A. HANAN, PHD California Department of Fish and Wildlife, Marine Region, 619 2nd Street Eureka, CA 95501, USA (EWR) Hanan and Associates, P.O. Box 8914 Rancho Santa Fe, CA 92067, USA (DAH) *Correspondent: [email protected] Key words: California scorpionfish, days at liberty, Floy FD-94,Scorpaena guttata, tag return During the four-year period from 21 November 2002 to 24 July 2006, we performed a mark-recapture study on nearshore groundfish off southern and central California (Hanan and Curry 2012). For the study, volunteer fishermen aboard chartered commercial passen- ger fishing vessels (CPFV) caught by hook and line, 32 species of groundfish (32,366 total fish), including 2,751 California Scorpionfish, Scorpaena guttata; these fish were marked with Floy FD-94 tags and released. As of the date of the Hanan and Curry paper, 257 scor- pionfish were reported as recaptured with an average days at liberty (DAL) of 408.8 days (431.6 SD; range 2 - 2,126 days). A total of 76 (33%) of these recaptured scorpionfish were recaptured within 1 km of their original tagging site, 155 (67%) were within 5 km, and 17 (1%) were recaptured at distances of 50 km or more from the original tagging site with a range of 68 to 1,788 DAL. On 21 November 2017, a tagged California scorpionfish was reported caught by Mr. Robert Rosenberg, a recreational angler, on a one-day trip aboard the CPFV New Del Mar out of Marina Del Rey, California. -
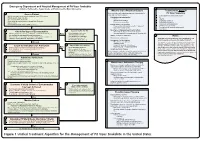
Snake Bite Protocol
Lavonas et al. BMC Emergency Medicine 2011, 11:2 Page 4 of 15 http://www.biomedcentral.com/1471-227X/11/2 and other Rocky Mountain Poison and Drug Center treatment of patients bitten by coral snakes (family Ela- staff. The antivenom manufacturer provided funding pidae), nor by snakes that are not indigenous to the US. support. Sponsor representatives were not present dur- At the time this algorithm was developed, the only ing the webinar or panel discussions. Sponsor represen- antivenom commercially available for the treatment of tatives reviewed the final manuscript before publication pit viper envenomation in the US is Crotalidae Polyva- ® for the sole purpose of identifying proprietary informa- lent Immune Fab (ovine) (CroFab , Protherics, Nash- tion. No modifications of the manuscript were requested ville, TN). All treatment recommendations and dosing by the manufacturer. apply to this antivenom. This algorithm does not con- sider treatment with whole IgG antivenom (Antivenin Results (Crotalidae) Polyvalent, equine origin (Wyeth-Ayerst, Final unified treatment algorithm Marietta, Pennsylvania, USA)), because production of The unified treatment algorithm is shown in Figure 1. that antivenom has been discontinued and all extant The final version was endorsed unanimously. Specific lots have expired. This antivenom also does not consider considerations endorsed by the panelists are as follows: treatment with other antivenom products under devel- opment. Because the panel members are all hospital- Role of the unified treatment algorithm -

Paralabrax Nebulifer) in Nearshore Waters Off Northern San Diego County
ROBERTS ET AL.: FEEDING HABITS OF BARRED SAND BASS CalCOFI Rep., Vol. XXV, 1984 THE FEEDING HABITS OF JUVENILE-SMALL ADULT BARRED SAND BASS (PARALABRAX NEBULIFER) IN NEARSHORE WATERS OFF NORTHERN SAN DIEGO COUNTY DALE A. ROBERTS‘, EDWARD E. DeMARTINI’, AND KENNETH M. PLUMMER2 Marine Science Institute University of California Santa Barbara, California 93106 ABSTRACT pelecipodos y peces epibent6nicos. Estas observa- The feeding habits of juvenile-small adult barred ciones no concuerdan con estudios previos, 10s cuales sand bass (Purulubrax nebulifer) are described, based consideran a la anchoveta del norte, Engruulis mor- on 165 specimens 123-523 mm standard length (SL) dux, como el elemento mas importante en la dieta de collected between San Onofre and Oceanside, Califor- P. nebulifer de tallas similares a las analizadas durante nia, at depths ranging from 8 to 30 m. Collections esta estudio. La dieta de P. nebulifer pequeiios (< 240 were made during an annual cycle from March 1981 to mm de longitud esthndar) es distinta debido a la pre- March 1982. sencia de crustaceos (misidaceos y antipodos gamir- The diet of the barred sand bass indicates that it idos), mientras que 10s ejemplares grandes (> 320 forages in close proximity to the substrate. Brachyuran mm LE) consumieron presas grandes como Porich- crabs, mysids, pelecypods, and epibenthic fishes were thys notutus (80-160 mm LE) y Octopus. P. nebulifer the most important prey. These findings are contrary de talla mediana (240-320 mm LE) contenian en su to previous studies, which found northern anchovy est6mago presas similares a las consumidas por 10s (Engruulis mordux) to be of major importance in the ejemplares grandes y pequeiios. -
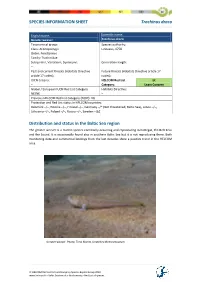
HELCOM Red List
SPECIES INFORMATION SHEET Trachinus draco English name: Scientific name: Greater weever Trachinus draco Taxonomical group: Species authority: Class: Actinopterygii Linnaeus, 1758 Order: Perciformes Family: Trachinidae Subspecies, Variations, Synonyms: Generation length: – Past and current threats (Habitats Directive Future threats (Habitats Directive article 17 article 17 codes): – codes): – IUCN Criteria: HELCOM Red List LC – Category: Least Concern Global / European IUCN Red List Category Habitats Directive: NE/NE – Previous HELCOM Red List Category (2007): VU Protection and Red List status in HELCOM countries: Denmark –/–, Estonia –/–, Finland –/–, Germany –/* (Not threatened, Baltic Sea), Latvia –/–, Lithuania –/–, Poland –/–, Russia –/–, Sweden –/LC Distribution and status in the Baltic Sea region The greater weever is a marine species commonly occurring and reproducing in Kattegat, the Belt Seas and the Sound. It is occasionally found also in southern Baltic Sea but it is not reproducing there. Both monitoring data and commercial landings from the last decades show a positive trend in the HELCOM area. Greater weaver. Photo: Timo Moritz, Deutches Meeresmuseum. © HELCOM Red List Fish and Lamprey Species Expert Group 2013 www.helcom.fi > Baltic Sea trends > Biodiversity > Red List of species SPECIES INFORMATION SHEET Trachinus draco Fig.1 Catch per unit effort (number per trawling hour) of greater weever in international bottom trawl surveys in Kattegat (IBTS) during third quarter of the year (Linear fit and correlation coefficient -

ABSTRACT MORRIS, JAMES ADIEL, JR. The
ABSTRACT MORRIS, JAMES ADIEL, JR. The Biology and Ecology of the Invasive Indo-Pacific Lionfish. (Under the direction of James A. Rice and John J. Govoni.) The Indo-Pacific lionfishes, Pterois miles and P. volitans, are now established along the Southeast U.S. and Caribbean and are expected to expand into the Gulf of Mexico and South America. Prior to this invasion little was known regarding the biology and ecology of these lionfishes. I provide a synopsis of lionfish biology and ecology including: invasion chronology, taxonomy, local abundance, reproduction, early life history and dispersal, venomology, feeding ecology, parasitology, potential impacts, and control and management. This information was collected by review of the literature and by direct field and experimental study. I confirm the existence of an unusual supraocular tentacle phenotype and suggest that the high prevalence of this phenotype in the Atlantic is not the result of selection, but likely ontogenetic change. To characterize the trophic impacts of lionfish, I report a comprehensive assessment of diet that describes lionfish as a generalist piscivore that preys on over 40 species of teleost comprising more than 20 families. Next, I use the histology of gonads to describe both oogenesis and reproductive dynamics of lionfish. Lionfish females mature at approximately 170 mm total length and reproduce several times per month throughout the entire calendar year off North Carolina and the Bahamas. To investigate predation, an important component of natural mortality, I assessed the vulnerability of juvenile lionfish to predation by native serranids. Juvenile lionfish were largely avoided as prey suggesting that predation mortality by serranids will not likely be a significant source of mortality for lionfish populations. -

Expressed Sequence Tags in Venomous Tissue of Scorpaena Plumieri (Scorpaeniformes: Scorpaenidae)
Neotropical Ichthyology, 12(4):871-878, 2014 Copyright © 2014 Sociedade Brasileira de Ictiologia DOI: 10.1590/1982-0224-20130149 Expressed sequence tags in venomous tissue of Scorpaena plumieri (Scorpaeniformes: Scorpaenidae) Fábio L. S. Costa1, Maria E. De Lima1, Adriano C. Pimenta1, Suely G. Figueiredo2, Evanguedes Kalapothakis3 and Carlos E. Salas4 Species of the family Scorpaenidae are responsible for accidents and sporadic casualties by the shore they inhabit. The species Scorpaena plumieri from this family populate the Northeastern and Eastern coast of Brazil causing human envenomation characterized by local and systemic symptoms. In experimental animals the venom induces cardiotoxic, hypotensive, and airway respiratory effects. As first step to identify the venom components we isolated gland mRNA to produce a cDNA library from the fish gland. This report describes the partial sequencing of 356 gland transcripts from S. plumieri. BLAST analysis of transcripts showed that 30% were unknown sequences, 17% hypothetical proteins, 17% related to metabolic enzymes, 14% belonged to signal transducing functions and the remaining groups (7-8%) composed by gene related with expressing proteins, regulatory proteins and structural proteins. A considerable number of these EST were not found in available databases suggesting the existence of new proteins and/or functions yet to be discovered. By screening the library with antibodies against a lectin fraction from S. plumieri venom we identified several clones whose DNA sequence showed similarities with lectins found in fish. In silico analysis of these clones confirm the identity of these molecules in the venom gland of S. plumieri. Espécies da família Scorpaenidae são responsáveis por acidentes e mortes esporádicas ao longo da costa que habitam. -

Fishes of Terengganu East Coast of Malay Peninsula, Malaysia Ii Iii
i Fishes of Terengganu East coast of Malay Peninsula, Malaysia ii iii Edited by Mizuki Matsunuma, Hiroyuki Motomura, Keiichi Matsuura, Noor Azhar M. Shazili and Mohd Azmi Ambak Photographed by Masatoshi Meguro and Mizuki Matsunuma iv Copy Right © 2011 by the National Museum of Nature and Science, Universiti Malaysia Terengganu and Kagoshima University Museum All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means without prior written permission from the publisher. Copyrights of the specimen photographs are held by the Kagoshima Uni- versity Museum. For bibliographic purposes this book should be cited as follows: Matsunuma, M., H. Motomura, K. Matsuura, N. A. M. Shazili and M. A. Ambak (eds.). 2011 (Nov.). Fishes of Terengganu – east coast of Malay Peninsula, Malaysia. National Museum of Nature and Science, Universiti Malaysia Terengganu and Kagoshima University Museum, ix + 251 pages. ISBN 978-4-87803-036-9 Corresponding editor: Hiroyuki Motomura (e-mail: [email protected]) v Preface Tropical seas in Southeast Asian countries are well known for their rich fish diversity found in various environments such as beautiful coral reefs, mud flats, sandy beaches, mangroves, and estuaries around river mouths. The South China Sea is a major water body containing a large and diverse fish fauna. However, many areas of the South China Sea, particularly in Malaysia and Vietnam, have been poorly studied in terms of fish taxonomy and diversity. Local fish scientists and students have frequently faced difficulty when try- ing to identify fishes in their home countries. During the International Training Program of the Japan Society for Promotion of Science (ITP of JSPS), two graduate students of Kagoshima University, Mr. -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -

Approach and Management of Spider Bites for the Primary Care Physician
Osteopathic Family Physician (2011) 3, 149-153 Approach and management of spider bites for the primary care physician John Ashurst, DO,a Joe Sexton, MD,a Matt Cook, DOb From the Departments of aEmergency Medicine and bToxicology, Lehigh Valley Health Network, Allentown, PA. KEYWORDS: Summary The class Arachnida of the phylum Arthropoda comprises an estimated 100,000 species Spider bite; worldwide. However, only a handful of these species can cause clinical effects in humans because many Black widow; are unable to penetrate the skin, whereas others only inject prey-specific venom. The bite from a widow Brown recluse spider will produce local symptoms that include muscle spasm and systemic symptoms that resemble acute abdomen. The bite from a brown recluse locally will resemble a target lesion but will develop into an ulcerative, necrotic lesion over time. Spider bites can be prevented by several simple measures including home cleanliness and wearing the proper attire while working outdoors. Although most spider bites cause only local tissue swelling, early species identification coupled with species-specific man- agement may decrease the rate of morbidity associated with bites. © 2011 Elsevier Inc. All rights reserved. More than 100,000 species of spiders are found world- homes and yards in the southwestern United States, and wide. Persons seeking medical attention as a result of spider related species occur in the temperate climate zones across bites is estimated at 50,000 patients per year.1,2 Although the globe.2,5 The second, Lactrodectus, or the common almost all species of spiders possess some level of venom, widow spider, are found in both temperate and tropical 2 the majority are considered harmless to humans. -

Pontinus Castor Poey, 1860 Frequent Synonyms / Misidentifications: Pontinus Pollux Poey, 1860 / None
click for previous page Scorpaeniformes: Scorpaenidae 1245 Pontinus castor Poey, 1860 Frequent synonyms / misidentifications: Pontinus pollux Poey, 1860 / None. FAO names: En - Longsnout scorpionfish; Fr - Rascasse longnez; Sp - Rascacio de fondo. Diagnostic characters: A snapper-like, spiny-headed scorpionfish with snout longer than eye diameter. Dor- sal fin with 12 spines and 9 1/2 to 11 1/2, but usually 10 1/2 soft rays (last split to base and counted as 1 1/2).No dorsal-fin spines especially elongated. Usually 17 pectoral-fin rays, all unbranched. Second preopercular spine small or absent.Scales ctenoid (rough to touch).Vertical scale rows 45 to 55.Colour: red or reddish pink on a pale background; some scattered dusky specks on body and head; fins spotted with red. Size: The largest reported specimen is 260 mm standard length. Habitat, biology, and fisheries: Predatory and bottom-dwelling, in depths of about 45 to 400 m, probably on rocky or shell bottom. Not subjected to a special fishery; caught incidentally as part of artisanal fisheries throughout its range. Caught mainly with hook-and-line and traps. Marketed fresh. Distribution: This species has been reported from depths of 45 to 180 m from Bermuda, the Bahamas, Cuba, Puerto Rico, and the Virgin Is- lands. It has yet to be reported more widely in the Caribbean. 1246 Bony Fishes Pontinus helena Eschmeyer, 1965 Frequent synonyms / misidentifications: None / None. FAO names: En - Helena scorpionfish. Diagnostic characters: Dorsal fin with 12 spines and 9 1/2 to 10 1/2 soft rays (last split to base and counted as 1 1/2). -

Parks Victoria Technical Series No
Deakin Research Online This is the published version: Barton, Jan, Pope, Adam and Howe, Steffan 2012, Marine protected areas of the Flinders and Twofold Shelf bioregions Parks Victoria, Melbourne, Vic. Available from Deakin Research Online: http://hdl.handle.net/10536/DRO/DU:30047221 Reproduced with the kind permission of the copyright owner. Copyright: 2012, Parks Victoria. Parks Victoria Technical Paper Series No. 79 Marine Natural Values Study (Vol 2) Marine Protected Areas of the Flinders and Twofold Shelf Bioregions Jan Barton, Adam Pope and Steffan Howe* School of Life & Environmental Sciences Deakin University *Parks Victoria August 2012 Parks Victoria Technical Series No. 79 Flinders and Twofold Shelf Bioregions Marine Natural Values Study EXECUTIVE SUMMARY Along Victoria’s coastline there are 30 Marine Protected Areas (MPAs) that have been established to protect the state’s significant marine environmental and cultural values. These MPAs include 13 Marine National Parks (MNPs), 11 Marine Sanctuaries (MSs), 3 Marine and Coastal Parks, 2 Marine Parks, and a Marine Reserve, and together these account for 11.7% of the Victorian marine environment. The highly protected Marine National Park System, which is made up of the MNPs and MSs, covers 5.3% of Victorian waters and was proclaimed in November 2002. This system has been designed to be representative of the diversity of Victoria’s marine environment and aims to conserve and protect ecological processes, habitats, and associated flora and fauna. The Marine National Park System is spread across Victoria’s five marine bioregions with multiple MNPs and MSs in each bioregion, with the exception of Flinders bioregion which has one MNP. -

Marine Fishes of the Azores: an Annotated Checklist and Bibliography
MARINE FISHES OF THE AZORES: AN ANNOTATED CHECKLIST AND BIBLIOGRAPHY. RICARDO SERRÃO SANTOS, FILIPE MORA PORTEIRO & JOÃO PEDRO BARREIROS SANTOS, RICARDO SERRÃO, FILIPE MORA PORTEIRO & JOÃO PEDRO BARREIROS 1997. Marine fishes of the Azores: An annotated checklist and bibliography. Arquipélago. Life and Marine Sciences Supplement 1: xxiii + 242pp. Ponta Delgada. ISSN 0873-4704. ISBN 972-9340-92-7. A list of the marine fishes of the Azores is presented. The list is based on a review of the literature combined with an examination of selected specimens available from collections of Azorean fishes deposited in museums, including the collection of fish at the Department of Oceanography and Fisheries of the University of the Azores (Horta). Personal information collected over several years is also incorporated. The geographic area considered is the Economic Exclusive Zone of the Azores. The list is organised in Classes, Orders and Families according to Nelson (1994). The scientific names are, for the most part, those used in Fishes of the North-eastern Atlantic and the Mediterranean (FNAM) (Whitehead et al. 1989), and they are organised in alphabetical order within the families. Clofnam numbers (see Hureau & Monod 1979) are included for reference. Information is given if the species is not cited for the Azores in FNAM. Whenever available, vernacular names are presented, both in Portuguese (Azorean names) and in English. Synonyms, misspellings and misidentifications found in the literature in reference to the occurrence of species in the Azores are also quoted. The 460 species listed, belong to 142 families; 12 species are cited for the first time for the Azores.