Supercritical Water Oxidation of Octol – Containing Wastewater
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cluster Weapons – Military Utility and Alternatives
FFI-rapport/2007/02345 Cluster weapons – military utility and alternatives Ove Dullum Forsvarets forskningsinstitutt/Norwegian Defence Research Establishment (FFI) 1 February 2008 FFI-rapport 2007/02345 Oppdrag 351301 ISBN 978-82-464-1318-1 Keywords Militære operasjoner / Military operations Artilleri / Artillery Flybomber / Aircraft bombs Klasevåpen / Cluster weapons Ammunisjon / Ammunition Approved by Ove Dullum Project manager Jan Ivar Botnan Director of Research Jan Ivar Botnan Director 2 FFI-rapport/2007/02345 English summary This report is made through the sponsorship of the Royal Norwegian Ministry of Foreign Affairs. Its purpose is to get an overview of the military utility of cluster munitions, and to find to which degree their capacity can be substituted by current conventional weapons or weapons that are on the verge of becoming available. Cluster munition roughly serve three purposes; firstly to defeat soft targets, i e personnel; secondly to defeat armoured of light armoured vehicles; and thirdly to contribute to the suppressive effect, i e to avoid enemy forces to use their weapons without inflicting too much damage upon them. The report seeks to quantify the effect of such munitions and to compare this effect with that of conventional weapons and more modern weapons. The report discusses in some detail how such weapons work and which effect they have against different targets. The fragment effect is the most important one. Other effects are the armour piercing effect, the blast effect, and the incendiary effect. Quantitative descriptions of such effects are usually only found in classified literature. However, this report is exclusively based on unclassified sources. The availability of such sources has been sufficient to get an adequate picture of the effect of such weapons. -

Simulated Rainfall-Driven Dissolution of TNT, Tritonal, Comp B and Octol Particles
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by UNL | Libraries University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln US Army Research U.S. Department of Defense 2009 Simulated Rainfall-Driven Dissolution of TNT, Tritonal, Comp B and Octol Particles Susan Taylor Cold Regions Research and Engineering Laboratory, 72 Lyme Road, Hanover, NH James H. Lever Cold Regions Research and Engineering Laboratory, 72 Lyme Road, Hanover, NH Jennifer Fadden Cold Regions Research and Engineering Laboratory, 72 Lyme Road, Hanover, NH Nancy Perron Cold Regions Research and Engineering Laboratory, 72 Lyme Road, Hanover, NH Bonnie Packer US Army Environmental Command, Hoadley Road, Aberdeen Proving Ground, MD 21010, United States Follow this and additional works at: https://digitalcommons.unl.edu/usarmyresearch Part of the Operations Research, Systems Engineering and Industrial Engineering Commons Taylor, Susan; Lever, James H.; Fadden, Jennifer; Perron, Nancy; and Packer, Bonnie, "Simulated Rainfall- Driven Dissolution of TNT, Tritonal, Comp B and Octol Particles" (2009). US Army Research. 52. https://digitalcommons.unl.edu/usarmyresearch/52 This Article is brought to you for free and open access by the U.S. Department of Defense at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in US Army Research by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Chemosphere 75 (2009) 1074–1081 Contents lists available at ScienceDirect -

Blast Effects Evaluation Using TNT Equivalent
50 Scientific Technical Review,Vol.LIX,No.3-4,2009 UDK: 662.1/4:541.42.6 COSATI: 19-01, 19-04 Blast Effects Evaluation Using TNT Equivalent Zoran Bajić, MSc (Eng)1) Jovica Bogdanov, MSc (Eng)1) Radun Jeremić, PhD (Eng)1) This paper shows blast parameters of high explosives commonly used in explosive ordnance of Serbian armed forces regarding TNT equivalents. Primary blast wave parameters, overpressure, impulse and positive phase duration are calculated using modified Sadovskiy equations and the modified K-B equation. TNT equivalents of the observed explosives are determined using thermochemical calculations based on BKW EOS parameters. The calculated blast wave parameters show significant influence of used explosives concerning the TNT equivalent. Key words: explosive materials, physics of explosion, blast wave, blast wave effect, thermochemical calculation, TNT equivalent, equation of state, Sadovskiy equations. Introduction (modified by the US DoD) for blast pressure and impulse FTER the detonation occurs, the ambient pressure determination using scaled distance is shown in eq.(4) [4]. A increases almost instantaneously and promptly begins 234 to decay, forming a nearly triangular overpressure pulse. PI,=++ exp( A B ln Z C (ln) Z + D (ln) Z + E (ln) Z ) (4) The peak pressure is called the peak positive overpressure. It represents the pressure seen at a point in space when the Where pressure is in kPa, impulse in Pa·s/kg1/3, Z is in shock wave is unimpeded in its motion. The duration of the m/kg1/3 and A, B, C, D and E empirical coefficients. positive overpressure is called the positive phase. -

Explosives Detector C04 User Manual
EXPLOSIVES DETECTOR C04 USER MANUAL HTBC.413444.001RE CONTENTS 1. INTRODUCTION 3 2. DESCRIPTION AND OPERATION 3 2.1. Product designation 3 2.2. Device specifications 3 2.3. Product components and accessories 5 2.4. Device design and operation 6 2.4.1. Principle of operation 6 2.4.2. Device description 6 2.4.3. Description of control buttons 10 2.4.4. Device menu description 11 2.5. Safety Precautions 20 3. INTENDED USE 21 3.1. Operability check 21 3.2. Explosives searching 22 3.3. False activation 25 4. THE BATTERY BLOCK CHANGING AND CHARGING 25 5. PIEZO DESORBER CHAMBER USE 28 6. TRANSPORTATION AND STORAGE 31 7. AFTER-SALE SERVICE 32 Appendix 1. Device maintenance 33 Appendix 2. Common troubles and solutions 36 1. INTRODUCTION This User Manual contains the necessary information on the components, internal design and operating principles of the Explosives Detector C04 (hereinafter referred to as the device) and information necessary for its correct and long-term service. 2. DESCRIPTION AND OPERATION 2.1. Product designation The device is designed to detect explosive vapors when examining various objects (personal belongings, baggage, mail, parcels, vehicles, premises, etc.), as well in search for trace amounts of explosive substances using a piezoelectric desorption chamber. 2.2. Device specifications The main specifications of the device are given in Table 1. Table 1. Main specifications. Parameter Parameter Unit value AC supply voltage rating 18 V Consumed current at the rated supply voltage (up to) 1,5 A Threshold for TNT detection in ambient air (up to) 10-14 g/cm3 Vortex sampling distance 60-100 mm Readiness time at warm temperature 5-60 sec at cold temperature Up to 600 Start time from stand-by mode 2-3 sec Analysis time (max) 2 sec Battery life in vapor mode, always on (min) 4 Battery life in trace mode, always on (min) 3 h Battery life in mixed mode: 5% in vapor mode, 5% in 10 trace mode, 90% in stand-by mode Dimensions, LxWxH (up to) 350x103x94 mm Parameter Unit Parameter value . -

Weathering of Explosives for Twenty Years
LA-11931 Weathering of Explosives for Twenty Years Los Alamos National Laboratory is operated by the University of California for the United States Department of Energy under contract W-7405-ENG-36. LA-11931 UC-741 Issued: May 1991 Weathering of Explosives for Twenty Years F. W. DuBois J. F. Baytos WEATHERING OF EXPLOSIVES FOR TWENTY YEARS F. W. DuBois and J. F. Baytos ABSTRACT Twelve high-explosive materials were buried in soil and exposed to the elements to determine their rate of disappearance from the environment. Only those explosives that contained TNT, barium nitrate, and boric acid disappeared at an environmentally significant rate. I. INTRODUCTION can be considered significant for their effective elimination from the environment. Experiments were undertaken to determine the persistence of explosives in the area surrounding a drop II. EXPERIMENTAL METHOD-SAMPLE tower at Los Alamos National Laboratory (Los Alamos) PREPARATION technical area TA-11, which is used in testing the sensitivity of explosives to impact. A location with soil, topography, Soil from the test site was screened through a l/4- by flora, and weather conditions typical of the area surrounding l/4-in. wire screen and dried to a moisture content of 0.25 the tower was selected. Soil was removed, mixed with wt%. Three sets of samples were prepared. explosives, replaced, and analyzed periodically for residual 1. The first set consisted of 12 samples, each containing explosives. Total time of the experiment was 20 years. 0.1 wt% of one of the 12 explosives. Five grams of Results after 4-l/2 years were reported in LA-4943 (June the powdered explosive was mixed with 5 kg of soil 1972)1 in a Patterson-Kelly twin-shell blender. -

Chemring Nobel Product Compositions – HMX
Chemring Nobel product compositions – HMX HMX Formulations Compositions Typical Product Specification Ingredients Weight % application HMX 49/49 Main charge HTA-3, Type I / Type II (Type TNT 29/28.65 MIL-E-46495 underwater II with CaSi added) Aluminium 22/22 explosive Calcium Silicate - /0.35 Octol 70/30, Type II, Class 1 HMX 70/75 MIL-O-45455 Shaped charges and 2/Octol 75/25, Type I TNT 30/25 HMX 98 H-764 US DWG No. 7259019 Calcium Recinate 1 Booster Graphite 1 HMX 95/94.5 Shaped, blast and HMX/Wax TL 1376-0825 Wax 5/4.5 fragmentation HMX/Wax/Graphite (OWC) Graphite - /1.0 charges HMX 98 HMX/Wax/Graphite (OWC) Customer specification Wax 1 Oil field perforators Graphite 1 HMX 95.5 LX-14 MIL-H-48358 Shaped charges Estane 4.5 HMX 96/96 Octastit VIII / VIIIG FS 1375-1626/1628 Plastic binder 4/3.9 Shaped charges Graphite - /0.1 HMX 86 Blast and PBXN-3 MIL-E-82738 Nylon 14 fragmentation HMX 95 PBXN-5 MIL-E-81111 Booster Viton 5 HMX 92 PBXN-9 MIL-E-82875 1,2 HyTemp 2 Shaped charges DOA 6 HMX 96 PBXW-11 DTL WS 33500 1,2 HyTemp 1 Shaped charges DOA 3 HMX 92 IM for shaped DPX-2 Chemring specification Hytemp 2 charges/ booster DOA 6 HMX 90 DPX-4 Chemring Specification Viton 10 Shaped charges HMX 64,4 HyTemp 1.4 DPX-5 Chemring Specification Enhanced blast DOA 4.2 Aluminium 30 HMX 50 Aluminium 46 DPX-6 Chemring specification Enhanced blast Hytemp 1 DOA 3 MIL-E-82895 HMX 97.05 Blast and CXM-9 (PBXN-112) (MIL-E-82919) Binder 2.95 fragmentation Chemring specification HMX 95 Shaped, blast and CXM-10 (PBXN-110) (MIL-DTL-82901A) IDP 5 fragment. -

IM Explosive for SMAW HEAA Warhead
IM Explosive for SMAW HEAA Warhead N. C. Johnson, C. W. Gonzalez, K. W. Reed, L. A. Kowalczyk, W. L. Myers, V. L. Beam, and V. A. Fields Indian Head Division, Naval Surface Warfare Center Indian Head, MD April 8, 2009 NDIA Gun and Missile Systems Conference DISTRIBUTION STATEMENT A. Approved for public release; distribution is unlimited. Presentation Outline • Objectives • Approach • System Description • Explosive Selection • Qualification and Performance Tests • Summary • Acknowledgements Objectives • Replace SMAW HEAA warhead fill (Octol) with explosive of comparable performance and improved IM characteristics – Sponsor directive: only system change will be explosive fill • Meet current HEAA penetration requirements • Qualify SMAW HEAA with IM warhead fill (SMAW HEAA-IM Warhead) Approach • Phase I: Explosive Selection – Explosive Selection Committee – IM and Performance Testing in SMAW HEAA Warhead – Downselection to Final Explosive Fill • Phase II: Qualification and Performance Testing SMAW HEAA-IM Warhead SMAW HEAA System Description • Shoulder-launched Multi-purpose Assault Weapon High Explosive Anti-Armor • DODIC HX06 • Effective against medium armor • SMAW HEAA consists of: – MK 153 MOD 0 Launcher – SMAW HEAA Encased Assault Rocket (EAR) • SMAW HEAA Rocket consists of: – Rocket motor – Impact fuze – Shaped charge, high explosive warhead Selection of IM Explosive Candidates Explosives Assessment • Explosive Output • IM Survivability • Safety & Reliability • Producibility / Life Cycle Costs Explosive Candidates – PBXN-9 • Used in Navy & Army shaped charge ordnance • Good IM in FCO/SCO/BI • Bad IM in FI – PBXN-11 • Better performance than PBXN-9 • Good IM in FCO/SCO • Bad IM in BI/FI – PBXW-114 • Equivalent performance to PBXN-110 • Good IM in FCO/SCO/BI • Potential for significant improvement in FI Explosive Properties Density, Explosive Composition Manufacture Method g/cc FCO/SCO/BI PBXN-9 HMX/binder pressed 1.73 V/V/V PBXN-11 HMX/binder pressed 1.80 V/V/IV PBXW-114 HMX/Al/binder cast 1.71 V/V/V melt (sedimentation) Octol HMX/TNT cast 1.82 I/I/V Phase I. -

Acceleration Characteristics of Discrete Fragments Generated from Explosively-Driven Cylindrical Metal Shells
materials Article Acceleration Characteristics of Discrete Fragments Generated from Explosively-Driven Cylindrical Metal Shells Mingxue Zhou , Cheng Wu *, Fengjiang An, Shasha Liao, Xiaoxia Yuan , Dongyu Xue and Jian Liu State Key Laboratory of Explosion Science and Technology, Beijing Institute of Technology, Beijing 100081, China; [email protected] (M.Z.); [email protected] (F.A.); [email protected] (S.L.); [email protected] (X.Y.); [email protected] (D.X.); [email protected] (J.L.) * Correspondence: [email protected]; Tel.: +86-01068915677 Received: 22 March 2020; Accepted: 26 April 2020; Published: 30 April 2020 Abstract: The acceleration characteristics of fragments generated from explosively-driven cylindrical shells are important issues in warhead design. However, there is as yet no reasonable theory for predicting the acceleration process of a specific metallic shell; existing approaches either ignore the effects of shell disintegration and the subsequent gas leakage on fragment acceleration or treat them in a simplified manner. In this paper, a theoretical model was established to study the acceleration of discrete fragments under the combined effect of shell disintegration and gas leakage. Firstly, an equation of motion was developed, where the acceleration of a cylindrical shell and the internal detonation gas was determined by the motive force impacting the inner surface of the metallic cylinder. To account for the force decrease induced by both the change in fragment area after the shell disintegrates and the subsequent drop in gas pressure due to gas leakage, the equation of motion was then associated with an equation for the locally isentropic expansion of the detonation gas and a modified gas-leakage equation. -

Simulated Rainfall-Driven Dissolution of TNT, Tritonal, Comp B and Octol Particles
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln US Army Research U.S. Department of Defense 2009 Simulated Rainfall-Driven Dissolution of TNT, Tritonal, Comp B and Octol Particles Susan Taylor Cold Regions Research and Engineering Laboratory, 72 Lyme Road, Hanover, NH James H. Lever Cold Regions Research and Engineering Laboratory, 72 Lyme Road, Hanover, NH Jennifer Fadden Cold Regions Research and Engineering Laboratory, 72 Lyme Road, Hanover, NH Nancy Perron Cold Regions Research and Engineering Laboratory, 72 Lyme Road, Hanover, NH Bonnie Packer US Army Environmental Command, Hoadley Road, Aberdeen Proving Ground, MD 21010, United States Follow this and additional works at: https://digitalcommons.unl.edu/usarmyresearch Part of the Operations Research, Systems Engineering and Industrial Engineering Commons Taylor, Susan; Lever, James H.; Fadden, Jennifer; Perron, Nancy; and Packer, Bonnie, "Simulated Rainfall- Driven Dissolution of TNT, Tritonal, Comp B and Octol Particles" (2009). US Army Research. 52. https://digitalcommons.unl.edu/usarmyresearch/52 This Article is brought to you for free and open access by the U.S. Department of Defense at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in US Army Research by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Chemosphere 75 (2009) 1074–1081 Contents lists available at ScienceDirect Chemosphere journal homepage: www.elsevier.com/locate/chemosphere Simulated rainfall-driven dissolution -

An Overview of Gurney Method for Estimating the Initial Velocities of Fragments for High Explosive Munition
Defense and Security Studies ISSN 2744-1741 Vol. 1, No. 1, December 2020, pp.16-25 Original Research https://doi.org/10.37868/dss.v1i1.72 An overview of Gurney method for estimating the initial velocities of fragments for high explosive munition Alan Catovic1* 1 Defense Technology Department, Mechanical Engineering Faculty, University of Sarajevo *[email protected] © The Author 2020. Abstract Published by The literature survey, related to the initial velocity of fragments for HE ARDA. ammunition is presented. The basic Gurney model for fragment initial velocity, that can be used for different munition configuration, is presented. The research we performed using the Gurney method for a different projectile types is given. Keywords: fragment velocity; Gurney constant; warhead detonation; 1. Introduction In the analysis of high explosive (HE) warheads with fragmentation, it is necessary to determine the initial velocity of the fragments, which is very important parameter of the terminal ballistics. Theoretical and experimental research has shown that the initial velocity of fragments formed by fragmentation of HE warheads depends on the ratio of explosive charge mass C and mass of warhead body M metal, as well as mechanical characteristics of warhead body material, type of explosive charge, and its detonation parameters. Fig. 1 shows a schematic representation of the HE warhead detonation process (controlled fragmentation). The mechanism of fragmentation is complex. The process of projectile expansion begins with the initiation of the primary explosive charge and proceeds with the detonation of the main charge, whereby the energy of the explosive is transformed very quickly from a potential form into mechanical work. -
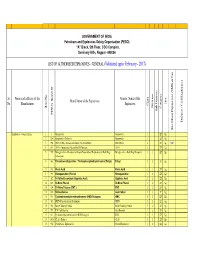
LIST of AUTHORISED EXPLOSIVES UNDER FINALISATION by Ankur
GOVERNMENT OF INDIA Petroleum and Explosives Safety Organisation (PESO) “A” Block, 5th Floor, CGO Complex, Seminary Hills, Nagpur- 440006 LIST OF AUTHORISED EXPLOSIVES - GENERAL (Validated upto February - 2017) Sr. Name and address of the Generic Name of the Brand Name of the Explosives Unit No. Manufacturer Explosives Class Division Item No. Category Sub Division Sub PESO's Brand ID Defence / Civil Explosives Site Mixed Explosives (SME) or Not or (SME) Mixed Explosives Site Explosives - General Items 1 1 Gun powder Gunpowder 1 ZZ Kg. 2 1204 Gunpowder (Defence) Gunpowder 1 ZZ Kg. 3 952 ANFO (SME) - Ammonium Nitrate Fuel Oil Mixture SME-ANFO 2 ZZ Kg. SME 4 811 ANFO - Ammonium Nitrate Fuel Oil Mixture ANFO 2 ZZ Kg. 5 969 Nitroglycerine – Precursor of Liquid Desensitised Explosives for Bulk Drug Nitroglycerine - Bulk Drug Precursor 3 1 ZZ Kg. Substances 6 843 Tetranitromethylaniline / Trinitrophenylmethylnitramine (Tetryl) Tetryl 3 2 ZZ Kg. 7 812 Picric Acid Picric Acid 3 2 ZZ Kg. 8 959 Nitroguanidine (Picrite) Nitroguanidine 3 2 ZZ Kg. 9 582 Tri-Nitro Recorcinol (Styphnic Acid) Styphnic Acid 3 2 ZZ Kg. 10 815 Di-Nitro Phenol Di-Nitro Phenol 3 2 ZZ Kg. 11 599 Tri-Nitro Toluene (TNT ) TNT 3 2 ZZ Kg. 12 816 Nitrocellulose Gun Cotton 3 2 Y Kg. 13 913 Cyclotetramethylenetetranitramine (HMX/Octogen) HMX 3 2 ZZ Kg. 14 581 PETN- Pentaerythritol Tetranitrate PETN 3 2 ZZ Kg. 15 946 Metal Cladding Powder Metal Cladding Powder 3 2 ZZ Kg. 16 957 TNT Cast Booster Cast Booster 3 2 ZZ Kg. 17 813 Cyclotrimethylenetrinitramine (RDX/Hexogen) RDX 3 2 ZZ Kg. -

Performance Comparison of Octol and PBXN-110 in a Shaped Charge Warhead R
- و "د ژ " Archive of SID ل د، ر ٢ ، ر ٣٨، ن ٩٧:ص ١١٢-١٠ ﻣﻘﺎﻳﺴﻪ ﻋﻤﻠﻜﺮد اﻛﺘﻮل و PBXN-110 در ﻳﻚ ﺳﺮﺟﻨﮕﻲ ﺧﺮج ﮔﻮد رﺿﺎ ﻛﺴﺎﻳﻲ1 ، اﺣﻤﺪ ﻣﻼﻳﻲ2* ، وﺣﻴﺪ اﻓﺸﺎري3 ، ﻋﻠﻲ ﺧﻴﺮآﺑﺎدي14 1و4- ﻛﺎرﺷﻨﺎس -2 دﻛﺘﺮا -3 ﻛﺎر ﺷﻨﺎس ارﺷﺪ داﻧﺸﮕﺎه اﻣﺎم ﺣﺴﻴﻦ (ع) (ﺗﺎرﻳﺦ وﺻﻮل: 13/8/ 96 ، ﺗﺎرﻳﺦ ﭘﺬﻳﺮش: 96/11/21) ﭼﻜﻴﺪه ﺗﺸﻜﻴﻞ ﺟﺖ ﺣﺎﺻﻞ از ﻓﺮوﭘﺎﺷﻲ ﻻﻳﻨﺮ در ﺳﺮﺟﻨﮕﻲ ﻫﺎي ﺧﺮج ﮔﻮد ﺗﺎ ﺣﺪ زﻳﺎدي ﺑﻪ ﻣﺸﺨﺼﺎت ﻣﺎده ﻣﻨﻔﺠﺮه ﺷﺎرژ ﺷﺪه در آن ﺑﺴﺘﮕﻲ دار. ﻫﺪف اﺻـﻠﻲ در اﻳﻦ ﺗﺤﻘﻴﻖ، ﻣﻘﺎﻳﺴﻪ اﺛﺮات ﺑﻪ ﻛﺎ رﮔﻴﺮي اﻛﺘﻮل و PBXN-110 در ﻋﻤﻠﻜﺮد ﻧﻮﻋﻲ ﺳﺮﺟﻨﮕﻲ ﺧﺮج ﮔﻮدي ﺧﺎص ﺑﻮده اﺳ. اﻳﻦ ﻣﻮاد در ﻳﻚ ﭘﻮﺳﺘﻪ آﻟﻮﻣﻴﻨﻴﻤﻲ ﺧﺮج ﮔﻮد ﺑﺎ ﻻﻳﻨﺮ ﻣﺴﻲ 35 درﺟﻪ و ﻇﺮﻓﻴﺖ 510 ﺳﺎﻧﺘﻲﻣﺘﺮ ﻣﻜﻌﺐ ﺷﺎرژ ﺷﺪﻧ. ﻧﺘﺎﻳﺞ ﺣﺎﺻﻞ از ﺷﺒﻴﻪ ﺳﺎزي ﺑﺎ ﻧﺮم اﻓﺰار LS-Dyna ﻧﺸﺎن داد ﻛﻪ ﻣـﺆﺛﺮﺗﺮﻳﻦ ﻓﺎﺻﻠﻪ ﺗﻮﻗﻒ اﻳﻦ ﺳﺮﺟﻨﮕﻲ ﺗﺤ ﻘﻴﻘﺎﺗﻲ، cm 50 اﺳ. ﺑﺮ اﺳﺎس اﻳﻦ ﺷﺒﻴﻪ ﺳﺎزي، ﺧﺮج اﻛﺘﻮل ﻧﻔﻮذ ﺑﻴﺸﺘﺮي ﻧﺴﺒﺖ ﺑﻪ PBXN-110 اﻳﺠﺎد ﻣﻲ ﻛﻨ. ﻫﻢﭼﻨـﻴ ﻦ ﻧﺘﺎﻳﺞ آزﻣﻮن ﻫﺎي ﺗﺠﺮﺑﻲ ﻫﻢ ﻧﺸﺎن داد ﻛﻪ ﻋﻤﻖ ﻧﻔﻮذ ﺳﺮﺟﻨﮕﻲ ﻫﺎي ﺣﺎوي اﻛﺘﻮل و PBXN-110 ﺑﻪ ﺗﺮﺗﻴﺐ 444 و 324 ﻣﻴﻠﻲﻣﺘﺮ اﺳـ ﺖ. ﺳـﺮﻋﺖ اﻧﻔﺠـﺎر و ﭼﮕﺎﻟﻲ ﭘﺎﻳﻴﻦ ﺗﺮ ﺧﺮج PBXN-110 ﻧﺴﺒﺖ ﺑﻪ اﻛﺘﻮل، ﻣﻮﺟﺐ ﻋﻤﻠﻜﺮد ﺿﻌﻴﻒ ﺗﺮ آن در ﺳﺮﺟﻨﮕﻲ ﻧﻔﻮذ ﻛﻨﻨﺪه ﻣﻲ ﺷﻮ. واژهﻫﺎي ﻛﻠﻴﺪي : ﺧﺮج ﮔﻮد، ﻧﻔﻮذ، اﻛﺘﻮل، PBXN-110 ، ﺷﺒﻴﻪ ﺳﺎزي . Performance Comparison of Octol and PBXN-110 in a Shaped Charge Warhead R. Kasaie, A. Mollaie *, V. Afshari, A. Kheyrabadi Imam Hossein University (Received:11/4/2017, Accepted: 2/10/2018) Abstract The jet formation caused by the collapse of a liner mainly depend on the characteristics of explosive loaded in the shaped charge warhead.