Identification of Naphthalene Carboxylase As a Prototype for The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(12) United States Patent (10) Patent No.: US 9,334,531 B2 Li Et Al
USOO933.4531B2 (12) United States Patent (10) Patent No.: US 9,334,531 B2 Li et al. (45) Date of Patent: *May 10, 2016 (54) NUCLECACIDAMPLIFICATION (56) References Cited U.S. PATENT DOCUMENTS (71) Applicant: LIFE TECHNOLOGIES 5,223,414 A 6/1993 Zarling et al. CORPORATION, Carlsbad, CA (US) 5,616,478 A 4/1997 Chetverin et al. 5,670,325 A 9/1997 Lapidus et al. (72) Inventors: Chieh-Yuan Li, El Cerrito, CA (US); 5,928,870 A 7/1999 Lapidus et al. David Ruff, San Francisco, CA (US); 5,958,698 A 9, 1999 Chetverinet al. Shiaw-Min Chen, Fremont, CA (US); 6,001,568 A 12/1999 Chetverinet al. 6,033,881 A 3/2000 Himmler et al. Jennifer O'Neil, Wakefield, MA (US); 6,074,853. A 6/2000 Pati et al. Rachel Kasinskas, Amesbury, MA (US); 6,306,590 B1 10/2001 Mehta et al. Jonathan Rothberg, Guilford, CT (US); 6.432,360 B1 8, 2002 Church Bin Li, Palo Alto, CA (US); Kai Qin 6,440,706 B1 8/2002 Vogelstein et al. Lao, Pleasanton, CA (US) 6,511,803 B1 1/2003 Church et al. 6,929,915 B2 8, 2005 Benkovic et al. (73) Assignee: Life Technologies Corporation, 7,270,981 B2 9, 2007 Armes et al. 7,282,337 B1 10/2007 Harris Carlsbad, CA (US) 7,399,590 B2 7/2008 Piepenburg et al. 7.432,055 B2 * 10/2008 Pemov et al. ................ 435/6.11 (*) Notice: Subject to any disclaimer, the term of this 7,435,561 B2 10/2008 Piepenburg et al. -

Complete Thesis
University of Groningen Omega transaminases: discovery, characterization and engineering Palacio, Cyntia Marcela IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document version below. Document Version Publisher's PDF, also known as Version of record Publication date: 2019 Link to publication in University of Groningen/UMCG research database Citation for published version (APA): Palacio, C. M. (2019). Omega transaminases: discovery, characterization and engineering. Rijksuniversiteit Groningen. Copyright Other than for strictly personal use, it is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), unless the work is under an open content license (like Creative Commons). The publication may also be distributed here under the terms of Article 25fa of the Dutch Copyright Act, indicated by the “Taverne” license. More information can be found on the University of Groningen website: https://www.rug.nl/library/open-access/self-archiving-pure/taverne- amendment. Take-down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Downloaded from the University of Groningen/UMCG research database (Pure): http://www.rug.nl/research/portal. For technical reasons the number of authors shown on this cover page is limited to 10 maximum. Download date: 01-10-2021 Omega transaminases: discovery, characterization and engineering Cyntia Marcela Palacio 2019 Cover design by Cyntia Palacio and Joris Goudsmits, adapted from a decorative mosaic artwork on the railway underpass on Moesstraat, Groningen. -

Physiology and Biochemistry of Aromatic Hydrocarbon-Degrading Bacteria That Use Chlorate And/Or Nitrate As Electron Acceptor
Invitation for the public defense of my thesis Physiology and biochemistry of aromatic hydrocarbon-degrading of aromatic and biochemistry Physiology bacteria that use chlorate and/or nitrate as electron acceptor as electron nitrate and/or use chlorate that bacteria Physiology and biochemistry Physiology and biochemistry of aromatic hydrocarbon-degrading of aromatic hydrocarbon- degrading bacteria that bacteria that use chlorate and/or nitrate as electron acceptor use chlorate and/or nitrate as electron acceptor The public defense of my thesis will take place in the Aula of Wageningen University (Generall Faulkesweg 1, Wageningen) on December 18 2013 at 4:00 pm. This defense is followed by a reception in Café Carré (Vijzelstraat 2, Wageningen). Margreet J. Oosterkamp J. Margreet Paranimphs Ton van Gelder ([email protected]) Aura Widjaja Margreet J. Oosterkamp ([email protected]) Marjet Oosterkamp (911 W Springfield Ave Apt 19, Urbana, IL 61801, USA; [email protected]) Omslag met flap_MJOosterkamp.indd 1 25-11-2013 5:58:31 Physiology and biochemistry of aromatic hydrocarbon-degrading bacteria that use chlorate and/or nitrate as electron acceptor Margreet J. Oosterkamp Thesis-MJOosterkamp.indd 1 25-11-2013 6:42:09 Thesis committee Thesis supervisor Prof. dr. ir. A. J. M. Stams Personal Chair at the Laboratory of Microbiology Wageningen University Thesis co-supervisors Dr. C. M. Plugge Assistant Professor at the Laboratory of Microbiology Wageningen University Dr. P. J. Schaap Assistant Professor at the Laboratory of Systems and Synthetic Biology Wageningen University Other members Prof. dr. L. Dijkhuizen, University of Groningen Prof. dr. H. J. Laanbroek, University of Utrecht Prof. -
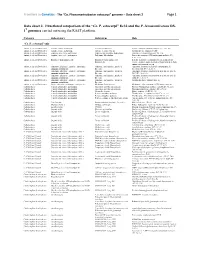
Ca. P. Ectocarpi” Ec32 and the P
Frontiers in Genetics - The “Ca. Phaeomarinobacter ectocarpi” genome – Data sheet 2 Page 1 Data sheet 2. Functional comparison of the “Ca. P. ectocarpi” Ec32 and the P. lavamentivorans DS- 1T genomes carried out using the RAST platform. Category Subcategory Subsystem Role “Ca. P. ectocarpi” only Amino Acids and Derivatives Alanine, serine, and glycine Alanine biosynthesis Valine--pyruvate aminotransferase (EC 2.6.1.66) Amino Acids and Derivatives Alanine, serine, and glycine Glycine cleavage system Sodium/glycine symporter GlyP Amino Acids and Derivatives Arginine; urea cycle, polyamines Arginine and Ornithine Degradation Ornithine cyclodeaminase (EC 4.3.1.12) Amino Acids and Derivatives Arginine; urea cycle, polyamines Polyamine Metabolism Putrescine transport ATP-binding protein PotA (TC 3.A.1.11.1) Amino Acids and Derivatives Branched-chain amino acids Branched-Chain Amino Acid Leucine-responsive regulatory protein, regulator for Biosynthesis leucine (or lrp) regulon and high-affinity branched-chain amino acid transport system Amino Acids and Derivatives Glutamine, glutamate, aspartate, asparagine; Glutamate and Aspartate uptake in Glutamate Aspartate periplasmic binding protein ammonia assimilation Bacteria precursor GltI (TC 3.A.1.3.4) Amino Acids and Derivatives Glutamine, glutamate, aspartate, asparagine; Glutamate and Aspartate uptake in Glutamate Aspartate transport system permease protein ammonia assimilation Bacteria GltJ (TC 3.A.1.3.4) Amino Acids and Derivatives Glutamine, glutamate, aspartate, asparagine; Glutamate and Aspartate -

(12) United States Patent (10) Patent No.: US 9,353,390 B2 Yan Et Al
US009353390B2 (12) United States Patent (10) Patent No.: US 9,353,390 B2 Yan et al. (45) Date of Patent: May 31, 2016 (54) GENETICALLY ENGINEERED MICROBES National Center for Biotechnology Information, National Library of AND METHODS FOR PRODUCING Medicine, National Institutes of Health, GenBank Locus 4-HYDROXYCOUMARIN NP 252920. Accession No. NP 252920, "isochorismate-pyruvate lyase IPseudomonas aeruginosa PAO1),” online). Bethesda, MD (71) Applicant: University of Georgia Research retrieved on Aug. 3, 2015. Retrieved from the Internet: <URL: Foundation, Inc., Athens, GA (US) http://www.ncbi.nlm.nih.gov/protein/NP 252920. 1; 3 pgs. National Center for Biotechnology Information, National Library of (72) Inventors: Yajun Yan, Athens, GA (US); Yuheng Medicine, National Institutes of Health, GenBank Locus Lin, Marietta, GA (US) NP 415125. Accession No. NP 415125. "isochorismate synthase 1 Escherichia coli str. K-12 substr. MG 1655, online. Bethesda, (73) Assignee: University of Georgia Research MD retrieved on Aug. 3, 2015). Retrieved from the Internet: <URL: http://www.ncbi.nlm.nih.gov/protein/NP 415125.1.>; 3 pgs. Foundation, Inc., Athens, GA (US) Ajikumar et al. “Isoprenoid Pathway Optimization for Taxol Precur (*) Notice: Subject to any disclaimer, the term of this sor Overproduction in Escherichia coli" Science, Oct. 1, 2010; 330(6000):70-4. patent is extended or adjusted under 35 Anthony et al. “Optimization of the mevalonate-based isoprenoid U.S.C. 154(b) by 0 days. biosynthetic pathway in Escherichia coli for production of the anti malarial drug precursor amorpha-4,11-diene' Metab. Eng. Jan. 2009; (21) Appl. No.: 14/304,105 11(1): 13-9. -

Structure of the Acetophenone Carboxylase Core Complex
www.nature.com/scientificreports OPEN Structure of the acetophenone carboxylase core complex: prototype of a new class of Received: 16 September 2016 Accepted: 24 November 2016 ATP-dependent carboxylases/ Published: 05 January 2017 hydrolases Sina Weidenweber1, Karola Schühle2, Ulrike Demmer1, Eberhard Warkentin1, Ulrich Ermler1 & Johann Heider2 Degradation of the aromatic ketone acetophenone is initiated by its carboxylation to benzoylacetate catalyzed by acetophenone carboxylase (Apc) in a reaction dependent on the hydrolysis of two ATP to ADP and Pi. Apc is a large protein complex which dissociates during purification into a heterooctameric Apc(αα′βγ)2 core complex of 482 kDa and Apcε of 34 kDa. In this report, we present the X-ray structure of the Apc(αα′βγ)2 core complex from Aromatoleum aromaticum at ca. 3 Å resolution which reveals a unique modular architecture and serves as model of a new enzyme family. Apcβ contains a novel domain fold composed of two β-sheets in a barrel-like arrangement running into a bundle of eight short polyproline (type II)-like helical segments. Apcα and Apcα′ possess ATP binding modules of the ASKHA superfamily integrated into their multidomain structures and presumably operate as ATP-dependent kinases for acetophenone and bicarbonate, respectively. Mechanistic aspects of the novel carboxylation reaction requiring massive structural rearrangements are discussed and criteria for specifically annotating the family members Apc, acetone carboxylase and hydantoinase are defined. Aromatic hydrocarbons are one of the most abundant classes of organic compounds in nature. They are primarily produced by plants as soluble secondary metabolic products or as components of the structural polymer lignin1. -

University of Oklahoma Graduate College the Role Of
UNIVERSITY OF OKLAHOMA GRADUATE COLLEGE THE ROLE OF GAMMAPROTEOBACTERIA IN AEROBIC ALKANE DEGRADATION IN OILFIELD PRODUCTION WATER FROM THE BARNETT SHALE A THESIS SUBMITTED TO THE GRADUATE FACULTY in partial fulfillment of the requirements for the Degree of MASTER OF SCIENCE BY MEREDITH MICHELLE THORNTON Norman, Oklahoma 2017 THE ROLE OF GAMMAPROTEOBACTERIA IN AEROBIC ALKANE DEGRADATION IN OILFIELD PRODUCTION WATER FROM THE BARNETT SHALE A THESIS APPROVED FOR THE DEPARTMENT OF MICROBIOLOGY AND PLANT BIOLOGY BY ______________________________________ Dr. Joseph Suflita, Chair ______________________________________ Dr. Kathleen Duncan ______________________________________ Dr. Amy Callaghan © Copyright by MEREDITH MICHELLE THORNTON 2017 All Rights Reserved. I would like to dedicate this work to my family. To my loving parents, Tim and Donna, who have sacrificed so much for me and continue to provide for and support my wildest aspirations. To my sister, Mackenzie, who inspires me to set an example worthy of following. And to my future husband, Clifford Dillon DeGarmo, who motivates me to work harder, dream bigger, and pursue the best version of myself. Acknowledgements This work would not be possible without the guidance and unwavering support of my advisor, Dr. Kathleen Duncan. Throughout this process, she has been a role model for me in more than just the academic setting; she has inspired me through her kind nature, diligent work ethic, and optimistic outlook on life. She invested so much time and effort into shaping me into a scientist and I can never express how thankful and lucky I am to have been part of her legacy. I would also like to acknowledge Drs. -

Comparative Genomics of Helicobacter Pylori
Schott et al. BMC Genomics 2011, 12:534 http://www.biomedcentral.com/1471-2164/12/534 RESEARCHARTICLE Open Access Comparative Genomics of Helicobacter pylori and the human-derived Helicobacter bizzozeronii CIII-1 strain reveal the molecular basis of the zoonotic nature of non-pylori gastric Helicobacter infections in humans Thomas Schott, Pradeep K Kondadi, Marja-Liisa Hänninen and Mirko Rossi* Abstract Background: The canine Gram-negative Helicobacter bizzozeronii is one of seven species in Helicobacter heilmannii sensu lato that are detected in 0.17-2.3% of the gastric biopsies of human patients with gastric symptoms. At the present, H. bizzozeronii is the only non-pylori gastric Helicobacter sp. cultivated from human patients and is therefore a good alternative model of human gastric Helicobacter disease. We recently sequenced the genome of the H. bizzozeronii human strain CIII-1, isolated in 2008 from a 47-year old Finnish woman suffering from severe dyspeptic symptoms. In this study, we performed a detailed comparative genome analysis with H. pylori, providing new insights into non-pylori Helicobacter infections and the mechanisms of transmission between the primary animal host and humans. Results: H. bizzozeronii possesses all the genes necessary for its specialised life in the stomach. However, H. bizzozeronii differs from H. pylori by having a wider metabolic flexibility in terms of its energy sources and electron transport chain. Moreover, H. bizzozeronii harbours a higher number of methyl-accepting chemotaxis proteins, allowing it to respond to a wider spectrum of environmental signals. In this study, H. bizzozeronii has been shown to have high level of genome plasticity. -
Biocatalytic C-C Bond Formation for One Carbon Resource Utilization
International Journal of Molecular Sciences Review Biocatalytic C-C Bond Formation for One Carbon Resource Utilization Qiaoyu Yang 1,2,3, Xiaoxian Guo 1,2, Yuwan Liu 1,2,* and Huifeng Jiang 1,2,* 1 Key Laboratory of Systems Microbial Biotechnology, Tianjin Institute of Industrial Biotechnology, Chinese Academy of Sciences, Tianjin 300308, China; [email protected] (Q.Y.); [email protected] (X.G.) 2 National Technology Innovation Center of Synthetic Biology, Tianjin 300308, China 3 University of Chinese Academy of Sciences, Beijing 100049, China * Correspondence: [email protected] (Y.L.); [email protected] (H.J.) Abstract: The carbon-carbon bond formation has always been one of the most important reactions in C1 resource utilization. Compared to traditional organic synthesis methods, biocatalytic C- C bond formation offers a green and potent alternative for C1 transformation. In recent years, with the development of synthetic biology, more and more carboxylases and C-C ligases have been mined and designed for the C1 transformation in vitro and C1 assimilation in vivo. This article presents an overview of C-C bond formation in biocatalytic C1 resource utilization is first provided. Sets of newly mined and designed carboxylases and ligases capable of catalyzing C-C bond formation for the transformation of CO2, formaldehyde, CO, and formate are then reviewed, and their catalytic mechanisms are discussed. Finally, the current advances and the future perspectives for the development of catalysts for C1 resource utilization are provided. Keywords: C1 resource utilization; carboxylases; C-C ligases; designed pathway Citation: Yang, Q.; Guo, X.; Liu, Y.; Jiang, H. -

Structural Classification and Properties of Ketoacyl Synthases and Biotin-Dependent Carboxylases Yingfei Chen Iowa State University
Iowa State University Capstones, Theses and Graduate Theses and Dissertations Dissertations 2012 Structural classification and properties of ketoacyl synthases and biotin-dependent carboxylases Yingfei Chen Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/etd Part of the Chemical Engineering Commons Recommended Citation Chen, Yingfei, "Structural classification and properties of ketoacyl synthases and biotin-dependent carboxylases" (2012). Graduate Theses and Dissertations. 12920. https://lib.dr.iastate.edu/etd/12920 This Thesis is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Graduate Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. Structural classification and properties of ketoacyl synthases and biotin-dependent carboxylases by Yingfei Chen A thesis submitted to the graduate faculty in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE Major: Chemical Engineering Program of Study Committee: Peter J. Reilly, Major Professor Ian C. Schneider Guang Song Iowa State University Ames, Iowa 2012 Copyright © Yingfei Chen, 2012. All rights reserved. 111 ii 111 Table of Contents Chapter 1: Introduction 1 Chapter 2: Literature review 5 Chapter 3: Structural classification and properties of ketoacyl synthases 22 Chapter 4: Classification of acyl-CoA and pyruvate carboxylases by their primary and 53 tertiary structures Chapter 5: Conclusions and future work 92 111 1 111 Chapter 1: Introduction The products of the fatty acid and polyketide synthesis systems are among the building blocks of life. -

Microorganisms and Methods for the Biosynthesis of Aromatics, 2,4-Pentadienoate and 1,3- Butadiene
(19) TZZ Z¥Z_T (11) EP 2 607 340 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: C07C 11/167 (2006.01) C12P 5/02 (2006.01) 26.06.2013 Bulletin 2013/26 C12N 15/52 (2006.01) C12P 7/16 (2006.01) C12N 1/15 (2006.01) C12N 1/19 (2006.01) (2006.01) (21) Application number: 13154607.9 C12N 1/21 (22) Date of filing: 26.07.2011 (84) Designated Contracting States: (72) Inventors: AL AT BE BG CH CY CZ DE DK EE ES FI FR GB • Osterhout, Robin E. GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO San Diego, CA 92121 (US) PL PT RO RS SE SI SK SM TR • Burgard, Anthony P. Designated Extension States: San Diego, CA 92121 (US) BA ME •Pharkya,Priti San Diego, CA 92121 (US) (30) Priority: 26.07.2010 US 367792 P •Burk,Mark J. 27.07.2010 US 368223 P San Diego, CA 92121 (US) 09.09.2010 US 381407 P (74) Representative: Jones Day (62) Document number(s) of the earlier application(s) in Rechtsanwälte,Attorneys- at-Law,Patentanwälte accordance with Art. 76 EPC: Prinzregentenstrasse 11 11740777.5 80538 München (DE) (71) Applicant: Genomatica, Inc. Remarks: San Diego, CA 92121 (US) This application was filed on 08-02-2013 as a divisional application to the application mentioned under INID code 62. (54) Microorganisms and methods for the biosynthesis of aromatics, 2,4-pentadienoate and 1,3- butadiene (57) The invention provides non-naturally occurring The invention additionally provides methods of using microbial organisms having a 1,3-butadiene pathway. -

Predictive Functional Profiling of Soil Microbes Under Different Tillages and Crop
Predictive Functional Profiling of Soil Microbes under Different Tillages and Crop Rotations in Ohio THESIS Presented in Partial Fulfillment of the Requirements for the Degree Master of Science in the Graduate School of The Ohio State University By Janani Hariharan Graduate Program in Environmental Science The Ohio State University 2015 Master's Examination Committee: Dr. Parwinder S. Grewal (Co-advisor) Dr. Warren A. Dick (Co-advisor) Dr. Margaret E. Staton Copyrighted by Janani Hariharan 2015 Abstract Food production and security is dependent on maintaining soil health and quality. Thus, the emphasis on sustainable and healthy soil function is a top priority for scientists and land managers. One of the most important factors that influences soil function is the microbial community. Recent advances have allowed us to quantify more accurately the composition of such communities, but there is still a knowledge gap with regard to the contribution of microorganisms to various processes occurring in the soil. Understanding this will facilitate the development of healthier agroecosystems. In this thesis, a predictive functional approach is used to elucidate bacterial species–function relationships. Bacterial community profiles were compared across two tillage systems and two crop rotations in Northern Ohio (Wooster and Hoytville). 16S rRNA gene-targeted sequencing was performed and the raw data obtained were filtered, denoised and processed using QIIME. Open-reference OTU picking and taxonomic assignment was performed using the Greengenes database. I then used a computational approach called PICRUSt (Phylogenetic Investigation of Communities by Reconstruction of Unobserved States) to predict metagenomes and the most likely functions performed by individual species of bacteria.