Comparative Analysis of Metagenomes from Three Methanogenic Hydrocarbon-Degrading Enrichment Cultures with 41 Environmental Samples
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Metagenomics Approaches for the Detection and Surveillance of Emerging and Recurrent Plant Pathogens
microorganisms Review Metagenomics Approaches for the Detection and Surveillance of Emerging and Recurrent Plant Pathogens Edoardo Piombo 1,2 , Ahmed Abdelfattah 3,4 , Samir Droby 5, Michael Wisniewski 6,7, Davide Spadaro 1,8,* and Leonardo Schena 9 1 Department of Agricultural, Forest and Food Sciences (DISAFA), University of Torino, 10095 Grugliasco, Italy; [email protected] 2 Department of Forest Mycology and Plant Pathology, Uppsala Biocenter, Swedish University of Agricultural Sciences, P.O. Box 7026, 75007 Uppsala, Sweden 3 Institute of Environmental Biotechnology, Graz University of Technology, Petersgasse 12, 8010 Graz, Austria; [email protected] 4 Department of Ecology, Environment and Plant Sciences, University of Stockholm, Svante Arrhenius väg 20A, 11418 Stockholm, Sweden 5 Department of Postharvest Science, Agricultural Research Organization (ARO), The Volcani Center, Rishon LeZion 7505101, Israel; [email protected] 6 U.S. Department of Agriculture—Agricultural Research Service (USDA-ARS), Kearneysville, WV 25430, USA; [email protected] 7 Department of Biological Sciences, Virginia Technical University, Blacksburg, VA 24061, USA 8 AGROINNOVA—Centre of Competence for the Innovation in the Agroenvironmental Sector, University of Torino, 10095 Grugliasco, Italy 9 Department of Agriculture, Università Mediterranea, 89122 Reggio Calabria, Italy; [email protected] * Correspondence: [email protected]; Tel.: +39-0116708942 Abstract: Globalization has a dramatic effect on the trade and movement of seeds, fruits and vegeta- bles, with a corresponding increase in economic losses caused by the introduction of transboundary Citation: Piombo, E.; Abdelfattah, A.; plant pathogens. Current diagnostic techniques provide a useful and precise tool to enact surveillance Droby, S.; Wisniewski, M.; Spadaro, protocols regarding specific organisms, but this approach is strictly targeted, while metabarcoding D.; Schena, L. -

(12) United States Patent (10) Patent No.: US 9,334,531 B2 Li Et Al
USOO933.4531B2 (12) United States Patent (10) Patent No.: US 9,334,531 B2 Li et al. (45) Date of Patent: *May 10, 2016 (54) NUCLECACIDAMPLIFICATION (56) References Cited U.S. PATENT DOCUMENTS (71) Applicant: LIFE TECHNOLOGIES 5,223,414 A 6/1993 Zarling et al. CORPORATION, Carlsbad, CA (US) 5,616,478 A 4/1997 Chetverin et al. 5,670,325 A 9/1997 Lapidus et al. (72) Inventors: Chieh-Yuan Li, El Cerrito, CA (US); 5,928,870 A 7/1999 Lapidus et al. David Ruff, San Francisco, CA (US); 5,958,698 A 9, 1999 Chetverinet al. Shiaw-Min Chen, Fremont, CA (US); 6,001,568 A 12/1999 Chetverinet al. 6,033,881 A 3/2000 Himmler et al. Jennifer O'Neil, Wakefield, MA (US); 6,074,853. A 6/2000 Pati et al. Rachel Kasinskas, Amesbury, MA (US); 6,306,590 B1 10/2001 Mehta et al. Jonathan Rothberg, Guilford, CT (US); 6.432,360 B1 8, 2002 Church Bin Li, Palo Alto, CA (US); Kai Qin 6,440,706 B1 8/2002 Vogelstein et al. Lao, Pleasanton, CA (US) 6,511,803 B1 1/2003 Church et al. 6,929,915 B2 8, 2005 Benkovic et al. (73) Assignee: Life Technologies Corporation, 7,270,981 B2 9, 2007 Armes et al. 7,282,337 B1 10/2007 Harris Carlsbad, CA (US) 7,399,590 B2 7/2008 Piepenburg et al. 7.432,055 B2 * 10/2008 Pemov et al. ................ 435/6.11 (*) Notice: Subject to any disclaimer, the term of this 7,435,561 B2 10/2008 Piepenburg et al. -

Complete Thesis
University of Groningen Omega transaminases: discovery, characterization and engineering Palacio, Cyntia Marcela IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document version below. Document Version Publisher's PDF, also known as Version of record Publication date: 2019 Link to publication in University of Groningen/UMCG research database Citation for published version (APA): Palacio, C. M. (2019). Omega transaminases: discovery, characterization and engineering. Rijksuniversiteit Groningen. Copyright Other than for strictly personal use, it is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), unless the work is under an open content license (like Creative Commons). The publication may also be distributed here under the terms of Article 25fa of the Dutch Copyright Act, indicated by the “Taverne” license. More information can be found on the University of Groningen website: https://www.rug.nl/library/open-access/self-archiving-pure/taverne- amendment. Take-down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Downloaded from the University of Groningen/UMCG research database (Pure): http://www.rug.nl/research/portal. For technical reasons the number of authors shown on this cover page is limited to 10 maximum. Download date: 01-10-2021 Omega transaminases: discovery, characterization and engineering Cyntia Marcela Palacio 2019 Cover design by Cyntia Palacio and Joris Goudsmits, adapted from a decorative mosaic artwork on the railway underpass on Moesstraat, Groningen. -

High-Throughput Pyrosequencing (To Use GS 20; Whole Genome Sequencer)
2007 International Meeting of the Microbiological Society of Korea >>> W1-1 High-throughput Pyrosequencing (to use GS 20; whole genome sequencer) Youseak Go Macrogen Corporation Genomic Analysis Division The Genome sequencer FLX system, developed by 454 Life Science Corporation, is an ultra-high-throughput automatic DNA sequencing system capable carrying out and monitoring sequencing reactions in a massively parallel fashion. Hundreds of thousands of simultaneous reactions, in the “well” of a PicoTiterPlate device. The basic chemistry utilize the release of pyrophosphate (PPi) that occurs with each nucleotide addition during DNA-directed DNA synthesis to generate an amount of light commensurate with the amount of PPi released; this light is captured by a charge-coupled device camera and converted into a digital signal. The raw data resulting from a sequencing run consists of a series of digital images captured by the camera, where the images are a representation of the surface of the PicoTiterPlate device over which the sequencing reaction are taking place; and each image corresponding to one reagent flow over that surface, as defined by the Run script. If the sample DNA fragment present in a given PicoTiterPlate well is extended during a nucleotide flow, light is emitted from the well and captured on the image corresponding to that flow. Furthermore, the amount of light emitted is proportional to the number of nucleotide extended. Knowledge of the nucleotide flowed while each image is being captured, of location on the PicoTiterPlate device where light is being emitted during each flow allows the software to identify PicoTiterPlate wells that contain a DNA library fragment and determine the sequence of the DNA fragments present in each well. -

Microbial Processes in Oil Fields: Culprits, Problems, and Opportunities
Provided for non-commercial research and educational use only. Not for reproduction, distribution or commercial use. This chapter was originally published in the book Advances in Applied Microbiology, Vol 66, published by Elsevier, and the attached copy is provided by Elsevier for the author's benefit and for the benefit of the author's institution, for non-commercial research and educational use including without limitation use in instruction at your institution, sending it to specific colleagues who know you, and providing a copy to your institution’s administrator. All other uses, reproduction and distribution, including without limitation commercial reprints, selling or licensing copies or access, or posting on open internet sites, your personal or institution’s website or repository, are prohibited. For exceptions, permission may be sought for such use through Elsevier's permissions site at: http://www.elsevier.com/locate/permissionusematerial From: Noha Youssef, Mostafa S. Elshahed, and Michael J. McInerney, Microbial Processes in Oil Fields: Culprits, Problems, and Opportunities. In Allen I. Laskin, Sima Sariaslani, and Geoffrey M. Gadd, editors: Advances in Applied Microbiology, Vol 66, Burlington: Academic Press, 2009, pp. 141-251. ISBN: 978-0-12-374788-4 © Copyright 2009 Elsevier Inc. Academic Press. Author's personal copy CHAPTER 6 Microbial Processes in Oil Fields: Culprits, Problems, and Opportunities Noha Youssef, Mostafa S. Elshahed, and Michael J. McInerney1 Contents I. Introduction 142 II. Factors Governing Oil Recovery 144 III. Microbial Ecology of Oil Reservoirs 147 A. Origins of microorganisms recovered from oil reservoirs 147 B. Microorganisms isolated from oil reservoirs 148 C. Culture-independent analysis of microbial communities in oil reservoirs 155 IV. -

Physiology and Biochemistry of Aromatic Hydrocarbon-Degrading Bacteria That Use Chlorate And/Or Nitrate As Electron Acceptor
Invitation for the public defense of my thesis Physiology and biochemistry of aromatic hydrocarbon-degrading of aromatic and biochemistry Physiology bacteria that use chlorate and/or nitrate as electron acceptor as electron nitrate and/or use chlorate that bacteria Physiology and biochemistry Physiology and biochemistry of aromatic hydrocarbon-degrading of aromatic hydrocarbon- degrading bacteria that bacteria that use chlorate and/or nitrate as electron acceptor use chlorate and/or nitrate as electron acceptor The public defense of my thesis will take place in the Aula of Wageningen University (Generall Faulkesweg 1, Wageningen) on December 18 2013 at 4:00 pm. This defense is followed by a reception in Café Carré (Vijzelstraat 2, Wageningen). Margreet J. Oosterkamp J. Margreet Paranimphs Ton van Gelder ([email protected]) Aura Widjaja Margreet J. Oosterkamp ([email protected]) Marjet Oosterkamp (911 W Springfield Ave Apt 19, Urbana, IL 61801, USA; [email protected]) Omslag met flap_MJOosterkamp.indd 1 25-11-2013 5:58:31 Physiology and biochemistry of aromatic hydrocarbon-degrading bacteria that use chlorate and/or nitrate as electron acceptor Margreet J. Oosterkamp Thesis-MJOosterkamp.indd 1 25-11-2013 6:42:09 Thesis committee Thesis supervisor Prof. dr. ir. A. J. M. Stams Personal Chair at the Laboratory of Microbiology Wageningen University Thesis co-supervisors Dr. C. M. Plugge Assistant Professor at the Laboratory of Microbiology Wageningen University Dr. P. J. Schaap Assistant Professor at the Laboratory of Systems and Synthetic Biology Wageningen University Other members Prof. dr. L. Dijkhuizen, University of Groningen Prof. dr. H. J. Laanbroek, University of Utrecht Prof. -

Pyrosequencing Technology for Short DNA Sequencing and Whole
生物物理 47(2),129-132(2007) 理論/実験 技術 Pyrosequencing Technology for Short DNA Sequencing and Whole Genome Sequencing Baback Gharizadeh1, Mehran Ghaderi2 and Pål Nyrén3 1Stanford Genome Technology Center, Stanford University 2Clinical Pathology/Cytology, Karolinska University Hospital 3Department of Biotechnology, Royal Institute of Technology 1.Introduction 2.Pyrosequencing chemistry Recent impressive advances in DNA sequencing technolo- Pyrosequencing technique is based on sequencing-by-syn- gies have accelerated the detailed analysis of genomes from thesis principle8), 9) and employs a series of four enzymes to ac- many organisms. We have been observing reports of complete curately detect short nucleic acid sequences during DNA syn- or draft versions of the genome sequence of several well-stud- thesis. In Pyrosequencing10), the sequencing primer is ied, multicellular organisms. Human biology and medicine hybridized to a single-stranded DNA biotin-labeled template are in the midst of a revolution by Human Genome Project1) (post-PCR alkali treated) and mixed with the enzymes; DNA as the main catalyst. polymerase, ATP sulfurylase, luciferase and apyrase, and the The chain termination sequencing method, also known as substrates adenosine 5′ phosphosulfate (APS) and luciferin. Sanger sequencing, developed by Frederick Sanger and col- Fig. 1 illustrates schematically the chemistry of Pyrose- leagues2), has been the most widely used sequencing method quencing method. Cycles of four deoxynucleotide triphos- since its advent in 1977 and still is in use after more than 29 phates (dNTPs) are separately added to the reaction mixture years since its advent. The remarkable advances in chemistry iteratively. After each nucleotide dispensation, DNA poly- and automation to the Sanger sequencing method has made it merase catalyzes the incorporation of complementary dNTP to a simple and elegant technique, central to almost all past into the template strand. -
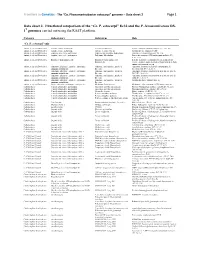
Ca. P. Ectocarpi” Ec32 and the P
Frontiers in Genetics - The “Ca. Phaeomarinobacter ectocarpi” genome – Data sheet 2 Page 1 Data sheet 2. Functional comparison of the “Ca. P. ectocarpi” Ec32 and the P. lavamentivorans DS- 1T genomes carried out using the RAST platform. Category Subcategory Subsystem Role “Ca. P. ectocarpi” only Amino Acids and Derivatives Alanine, serine, and glycine Alanine biosynthesis Valine--pyruvate aminotransferase (EC 2.6.1.66) Amino Acids and Derivatives Alanine, serine, and glycine Glycine cleavage system Sodium/glycine symporter GlyP Amino Acids and Derivatives Arginine; urea cycle, polyamines Arginine and Ornithine Degradation Ornithine cyclodeaminase (EC 4.3.1.12) Amino Acids and Derivatives Arginine; urea cycle, polyamines Polyamine Metabolism Putrescine transport ATP-binding protein PotA (TC 3.A.1.11.1) Amino Acids and Derivatives Branched-chain amino acids Branched-Chain Amino Acid Leucine-responsive regulatory protein, regulator for Biosynthesis leucine (or lrp) regulon and high-affinity branched-chain amino acid transport system Amino Acids and Derivatives Glutamine, glutamate, aspartate, asparagine; Glutamate and Aspartate uptake in Glutamate Aspartate periplasmic binding protein ammonia assimilation Bacteria precursor GltI (TC 3.A.1.3.4) Amino Acids and Derivatives Glutamine, glutamate, aspartate, asparagine; Glutamate and Aspartate uptake in Glutamate Aspartate transport system permease protein ammonia assimilation Bacteria GltJ (TC 3.A.1.3.4) Amino Acids and Derivatives Glutamine, glutamate, aspartate, asparagine; Glutamate and Aspartate -

Multiplexed Microsatellite Recovery Using Massively Parallel Sequencing
Molecular Ecology Resources (2011) 11, 1060–1067 doi: 10.1111/j.1755-0998.2011.03033.x Multiplexed microsatellite recovery using massively parallel sequencing T. N. JENNINGS,* B. J. KNAUS,* T. D. MULLINS,† S. M. HAIG† and R. C. CRONN* *Pacific Northwest Research Station, USDA Forest Service, 3200 SW Jefferson Way, Corvallis, OR 97331, USA, †Forest and Rangeland Ecosystem Science Center, US Geological Survey, 3200 SW Jefferson Way, Corvallis, OR 97331, USA Abstract Conservation and management of natural populations requires accurate and inexpensive genotyping methods. Traditional microsatellite, or simple sequence repeat (SSR), marker analysis remains a popular genotyping method because of the com paratively low cost of marker development, ease of analysis and high power of genotype discrimination. With the availabil ity of massively parallel sequencing (MPS), it is now possible to sequence microsatellite-enriched genomic libraries in multiplex pools. To test this approach, we prepared seven microsatellite-enriched, barcoded genomic libraries from diverse taxa (two conifer trees, five birds) and sequenced these on one lane of the Illumina Genome Analyzer using paired-end 80-bp reads. In this experiment, we screened 6.1 million sequences and identified 356 958 unique microreads that contained di- or trinucleotide microsatellites. Examination of four species shows that our conversion rate from raw sequences to polymorphic markers compares favourably to Sanger- and 454-based methods. The advantage of multiplexed MPS is that the staggering capacity of modern microread sequencing is spread across many libraries; this reduces sample preparation and sequencing costs to less than $400 (USD) per species. This price is sufficiently low that microsatellite libraries could be prepared and sequenced for all 1373 organisms listed as ‘threatened’ and ‘endangered’ in the United States for under $0.5 M (USD). -

Review Christopher P
4618 Electrophoresis 2008, 29, 4618–4626 Daniel G. Hert1 Review Christopher P. Fredlake1 1,2Ã Annelise E. Barron Advantages and limitations of next- 1Department of Chemical and Biological Engineering, generation sequencing technologies: Northwestern University, Evanston IL, USA A comparison of electrophoresis and 2Department of Bioengineering, Stanford University, Stanford, non-electrophoresis methods CA, USA The reference human genome provides an adequate basis for biological researchers to study the relationship between genotype and the associated phenotypes, but a large push Received July 14, 2008 is underway to sequence many more genomes to determine the role of various specifi- Revised August 29, 2008 Accepted August 29, 2008 cities among different individuals that control these relationships and to enable the use of human genome data for personalized and preventative healthcare. The current elec- trophoretic methodology for sequencing an entire mammalian genome, which includes standard molecular biology techniques for genomic sample preparation and the separation of DNA fragments using capillary array electrophoresis, remains far too expensive ($5 million) to make genome sequencing ubiquitous. The National Human Genome Research Institute has put forth goals to reduce the cost of human genome sequencing to $100 000 in the short term and $1000 in the long term to spur the innovative development of technologies that will permit the routine sequencing of human genomes for use as a diagnostic tool for disease. Since the announcement of these goals, several companies have developed and released new, non-electrophoresis- based sequencing instruments that enable massive throughput in the gathering of genomic information. In this review, we discuss the advantages and limitations of these new, massively parallel sequencers and compare them with the currently developing next generation of electrophoresis-based genetic analysis platforms, specifically microchip electrophoresis devices, in the context of three distinct types of genetic analysis. -

Community Analysis of Plant Biomass-Degrading Microorganisms from Obsidian Pool, Yellowstone National Park
Microb Ecol DOI 10.1007/s00248-014-0500-8 ENVIRONMENTAL MICROBIOLOGY Community Analysis of Plant Biomass-Degrading Microorganisms from Obsidian Pool, Yellowstone National Park Tatiana A. Vishnivetskaya & Scott D. Hamilton-Brehm & Mircea Podar & Jennifer J. Mosher & Anthony V. Palumbo & Tommy J. Phelps & Martin Keller & James G. Elkins Received: 12 May 2014 /Accepted: 16 September 2014 # Springer Science+Business Media New York (outside the USA) 2014 Abstract The conversion of lignocellulosic biomass into (OBP), was examined for potential biomass-active microorgan- biofuels can potentially be improved by employing robust mi- isms using cultivation-independent and enrichment techniques. croorganisms and enzymes that efficiently deconstruct plant Analysis of 33,684 archaeal and 43,784 bacterial quality-filtered polysaccharides at elevated temperatures. Many of the geother- 16S rRNA gene pyrosequences revealed that archaeal diversity mal features of Yellowstone National Park (YNP) are surrounded in the main pool was higher than bacterial; however, in the by vegetation providing a source of allochthonic material to vegetated area, overall bacterial diversity was significantly support heterotrophic microbial communities adapted to utilize higher. Of notable interest was a flooded depression adjacent to plant biomass as a primary carbon and energy source. In this OBP supporting a stand of Juncus tweedyi, a heat-tolerant rush study, a well-known hot spring environment, Obsidian Pool commonly found growing near geothermal features in YNP. The microbial community from heated sediments surrounding the The submitted manuscript has been authored by a contractor of the U.S. plants was enriched in members of the Firmicutes including Government under contract DE-AC05-00OR22725. Accordingly, the potentially (hemi)cellulolytic bacteria from the genera U.S. -

Pyrosequencing- Principles and Applications
Review Article ISSN 2250-0480 Vol 2/Issue 2/Apr-Jun 2012 PYROSEQUENCING- PRINCIPLES AND APPLICATIONS MD. FAKRUDDIN1*, ABHIJIT CHOWDHURY1, MD. NUR HOSSAIN1, KHANJADA SHAHNEWAJ BIN MANNAN2, REAZ MOHAMMAD MAZUMDAR3 1Institute of Food Science and Technology (IFST), Bangladesh Council of Scientific and Industrial Research (BCSIR), Dhaka, B angladesh. 2Center for Food & Waterborne Disease (CFWD), icddr'b, Dhaka, Bangladesh. 3BCSIR Laboratories Chittagong, Bangladesh Council of Scientific and Industrial Research (BCSIR), Bangladesh. ABSTRACT Pyrosequencing is the first alternative to the conventional Sanger method for de novo DNA sequencing. Pyrosequencing is a DNA sequencing technology based on the sequencing-by-synthesis principle. It employs a series of four enzymes to accurately detect nucleic acid sequences during the synthesis. Pyrosequencing has the potential advantages of accuracy, flexibility, parallel processing, and can be easily automated. Furthermore, the technique dispenses with the need for labeled primers, labeled nucleotides, and gel-electrophoresis. Pyrosequencing has opened up new possibilities for performing sequence-based DNA analysis. The method has been proven highly suitable for single nucleotide polymorphism analysis and sequencing of short stretches of DNA. Pyrosequencing has been successful for both confirmatory sequencing and de novo sequencing. By increasing the read length to higher scores and by shortening the sequence reaction time per base calling, pyrosequencing may take over many broad areas of DNA sequencing applications as the trend is directed to analysis of fewer amounts of specimens and large-scale settings, with higher throughput and lower cost. This article considers key features regarding different aspects of pyrosequencing technology, including the general principles, enzyme properties, sequencing modes, instrumentation, limitations, potential and future applications.